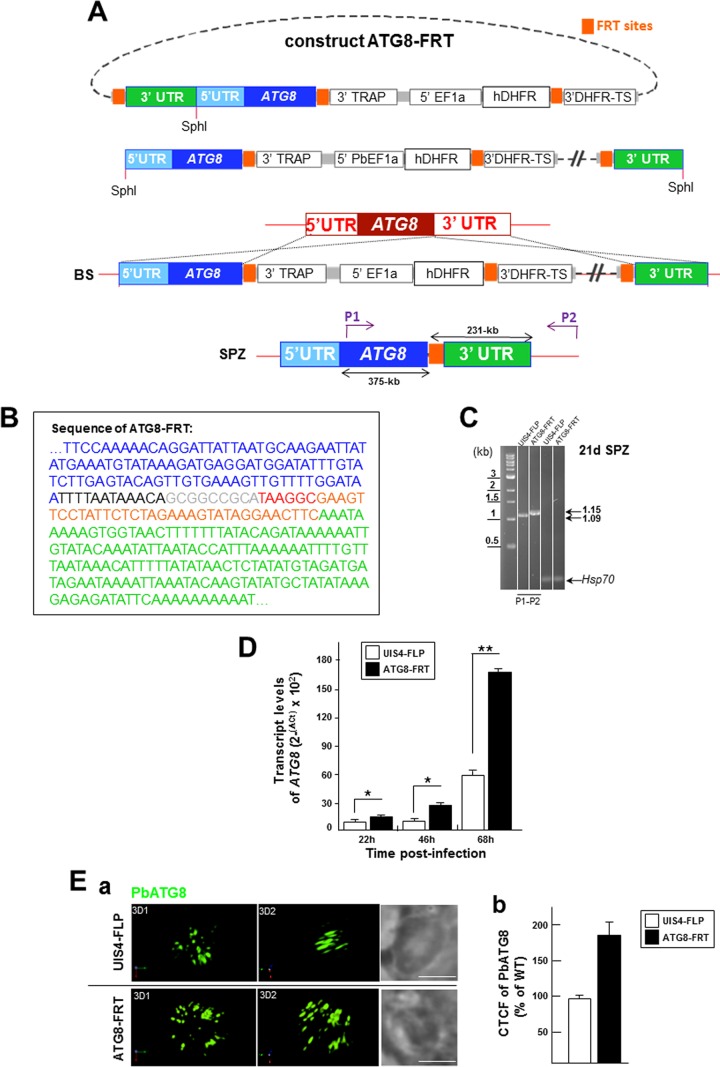
FIG 4 .
Generation of a parasite strain expressing ATG8 with altered 3′ UTR. (A) Schematic representation of the construct ATG8-FRT. (B and C) The plasmid pUC18-p3′TRAP-hDHFR containing two FRT sites was used as the template for insertion of ATG8 and the UTRs before linearization with SphI and transfection into a UIS4-FLP parasite strain to generate a parasite strain expressing PbATG8 with a modified 3′ region, which was characterized by the presence of extra nucleotides inserted before the 3′ UTR of ATG8 as shown in panel B (blue, end of the ATG8 gene; black, 12 bp of 3′ UTR of TRAP; gray, 9 bp of NotI site; red, 6 bp from p3′TRAP-hDHFR flirted; orange, 34 bp from the FRT site; green, beginning of the 3′ UTR of ATG8) and confirmed by PCR, as shown in panel C in UIS4-FLP (parental) and ATG8-FRT (3′ mutant) strains. The P1/P2 primers were used to verify the presence of extra nucleotides in the ATG8-FRT strain. The expected sizes of the fragments are shown to the right of the gels. SPZ, sporozoites. (D) ATG8 gene expression measured by qRT-PCR. The α-tubulin 1 gene was used as a reference to normalize the amounts of the ATG8 transcripts. Transcript levels were represented as 2−ΔΔCT × 100 to show levels of transcripts expressed comparatively in the parental and mutant strains. (E) (a) IFA on P. berghei-infected cells using anti-PbATG8 antibodies (green) 17 h p.i. Shown are two rotated views of a PV from the parental and mutant strains. Bars, 4 µm. (b) Quantification of PbATG8 fluorescence levels. The corrected total cell fluorescence (CTCF) level was calculated from PVs with wild-type (WT), UIS4-FLP, and ATG8-FRT parasites immunostained for PbATG8 (n = 23 to 31 PVs), and data for the two strains are expressed as percentages of wild-type fluorescence levels.