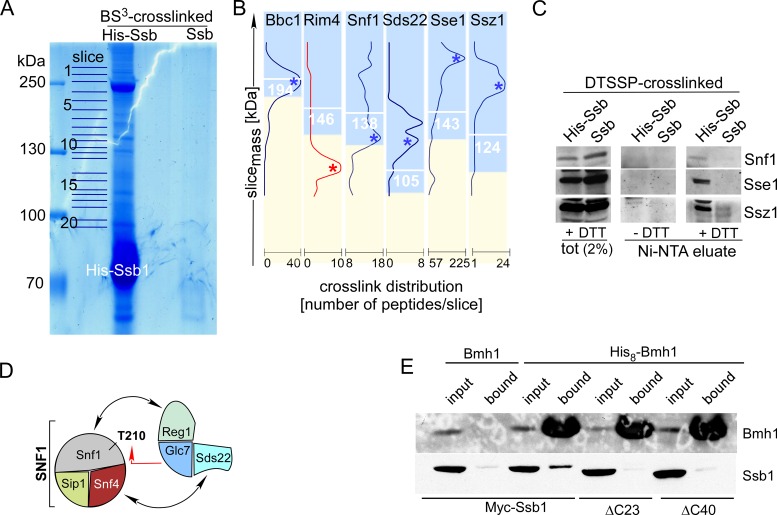
Figure 3.
Ssb1 directly interacts with all subunits of the SNF1 complex, Glc7, Reg1 and Bmh1. (A) Purified His-Ssb crosslink products. His-Ssb was crosslinked with BS3 and was subsequently purified via Ni-NTA under denaturing conditions (‘Materials and Methods’ section). Untagged Ssb treated the same way is shown as a control. Slices of the Coomassie-stained gel employed for mass spec analysis are indicated. (B) Crosslink distribution profiles. Ssb-crosslinks were selected if a major peak of peptides was observed in the molecular mass range ≥85% of the calculated mass of the Ssb-crosslink in kDa (white numbers). Profiles of potential Ssb-interactors are shown in blue. An example profile (Rim4), which was discarded from the list of potential interactors (single maximum ≤ 85%) is shown in red (Supplementary Table S4). (C) Identification of representative Ssb-crosslinks via immunodetection. After crosslinking with the cleavable crosslinker DTSSP His-Ssb1 or, as a control, Ssb was purified under denaturing, non-reducing conditions via Ni-NTA. Aliquots of the total and of the affinity purified Ssb-crosslinks were separated via SDS-PAGE under reducing (+DTT) or non-reducing (−DTT) conditions. Subsequently immunoblots were decorated with antibodies directed against Snf1, Sse1 and Ssz1. Shown is the molecular mass range of the immunoblots at which the respective Ssb-interactor migrates after cleavage of the disulfide bond within DTSSP (+DTT). (D) Subunits of the SNF1 and Glc7 complexes, which formed crosslinks with Ssb. Interactions previously reported (see ‘Results’ section) are indicated by black arrows. The red arrow indicates the interaction between Snf1 and Glc7, which occurs during dephosphorylation of Snf1-T210. (E) Bmh1 interacts with the C-terminus of Ssb. His8-Bmh1 was purified via Ni-NTA under native conditions, untagged Bmh1 served as a control. Strains expressed N-terminally Myc-tagged Ssb, Ssb-Δ23 or Ssb-Δ40 as indicated. Input (0.66%) and the material bound to Ni-NTA (100%) was analyzed via immunoblotting using antibodies directed against Bmh1 or the Myc-tag (Supplementary Figure S1C). Background binding of Ssb was probably due to general stickiness of the chaperone, or was due to the association of Ssb with proteins like Snf1, which contain internal poly-histidine stretches (Supplementary Figures S1A and S4, and Table S4).