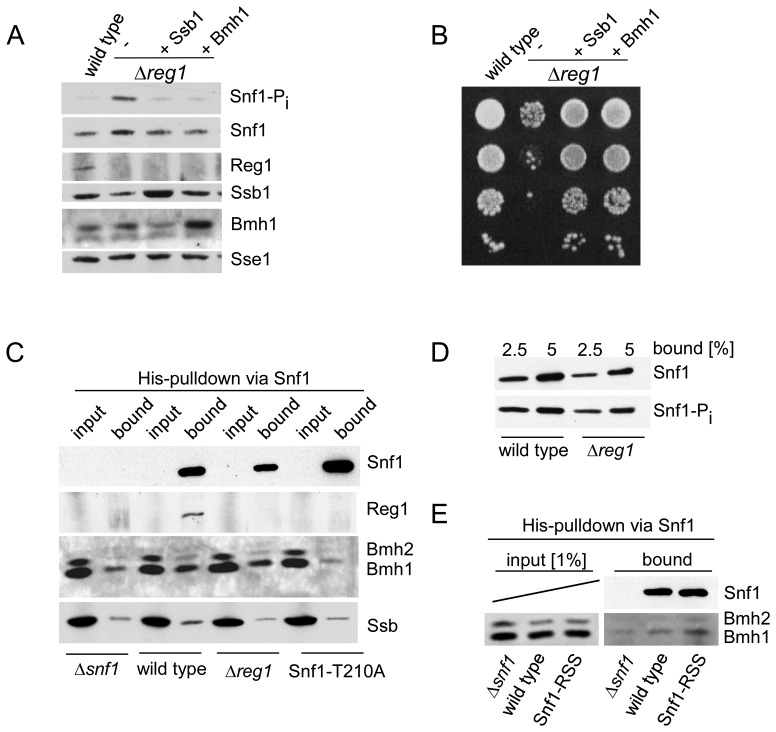
Figure 4.
Bmh suppresses growth defects of a Δreg1 strain and interacts with Snf1-T210-Pi. (A) Over-expression of Bmh1 suppresses growth defects of a Δreg1 strain. Phosphorylation of Snf1-T210 in glucose-grown wild-type, Δreg1 and Δreg1 over-expressing Ssb1 or Bmh1. Logarithmically growing yeast cultures were boiled prior to harvest and extract preparation to avoid harvest-induced phosphorylation of Snf1. Aliquots of total cell extracts were analyzed via immunoblotting with the antibodies indicated. Snf1 phosphorylation on Thr210 (Snf1-Pi) was analyzed using an antibody specifically recognizing the phosphorylated form of Snf1-T210. Sse1 served as a loading control. (B) Suppression of growth defects caused by the Δreg1 mutation. Serial 10-fold dilutions of log-phase cultures containing the same number of cells were spotted onto YPD plates and were incubated at 30°C for 2 days. (C) Bmh interacts with Snf1-T210-Pi. Cell extracts were prepared without boiling to maintain protein–protein interactions. Snf1, which contains a poly-histidine segment (Supplementary Figure S1A), was purified via Ni-NTA under native conditions, a Δsnf1 strain served as a control. Total extract (input) and affinity purified Snf1 (bound) from wild-type, Δreg1 and Snf1-T210A strains was analyzed via immunoblotting using antibodies directed against Snf1, Bmh, Reg1 and Ssb. The input corresponds to 0.33% of total extract added to the Ni-NTA pull down reaction. (see also Supplementary Figure S4). (D) Phosphorylation of Snf1-T210 occurs during cell harvest and extract preparation. Affinity purified Snf1 from wild-type and Δreg1 strains prepared without boiling (2.5 and 5% of the material shown in panel C) was analyzed for Snf1 and Snf1-Pi via immunoblotting. (E) Bmh interacts with the Snf1-RSS mutant. The experiment was performed and analyzed as described in panel C.