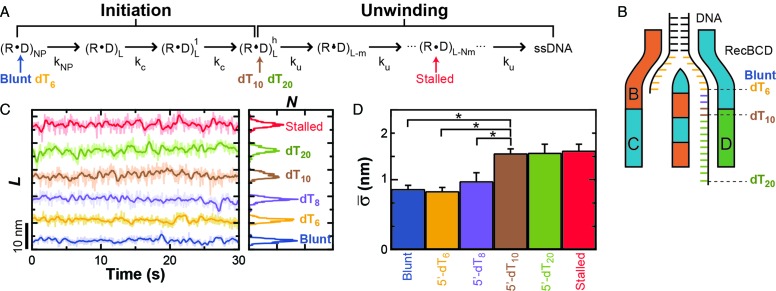
Figure 3.
Onset of conformational dynamics is coincident with entering an unwinding-competent state. (A) Kinetic diagram for RecBCD unwinding adapted from Wu and Lohman (25). Labels are defined as: RecBCD (R), DNA (D), non-productive state (NP), DNA length (L), size of step (m), number of steps (N), rate of slow isomerization (kNP), rate of initiation (kc) and rate of unwinding (ku). The unwinding-competent state is reached once the RecBCD–DNA complex is initiated. Initiation requires either ATP-dependent movement of RecBCD or a DNA substrate with a pre-existing single-stranded 5′ DNA tail of 10 thymidines, denoted 5′-(dT)10. Different tailed-DNA constructs (Blunt, dT6, dT8, dT10, dT20) positioned the RecBCD–DNA complex at different states within this kinetic diagram in the absence of added ATP (Indicated by arrows colored to indicate the length of the dT-tail). A stalled complex has unwound DNA and is thus positioned on the diagram to indicate such unwinding. (B) Cartoon of RecBCD bound to the tailed-DNA ends. (C) DNA length measurement for RecBCD bound to the following DNA substrates: blunt-ended (blue), 5′-(dT)6 (orange), 5′-(dT)8 (purple), 5′-(dT)10 (brown), 5′-(dT)20 (light green) and partially unwound (red). (D) Bar graph of the mean standard deviation for the conditions in (C).