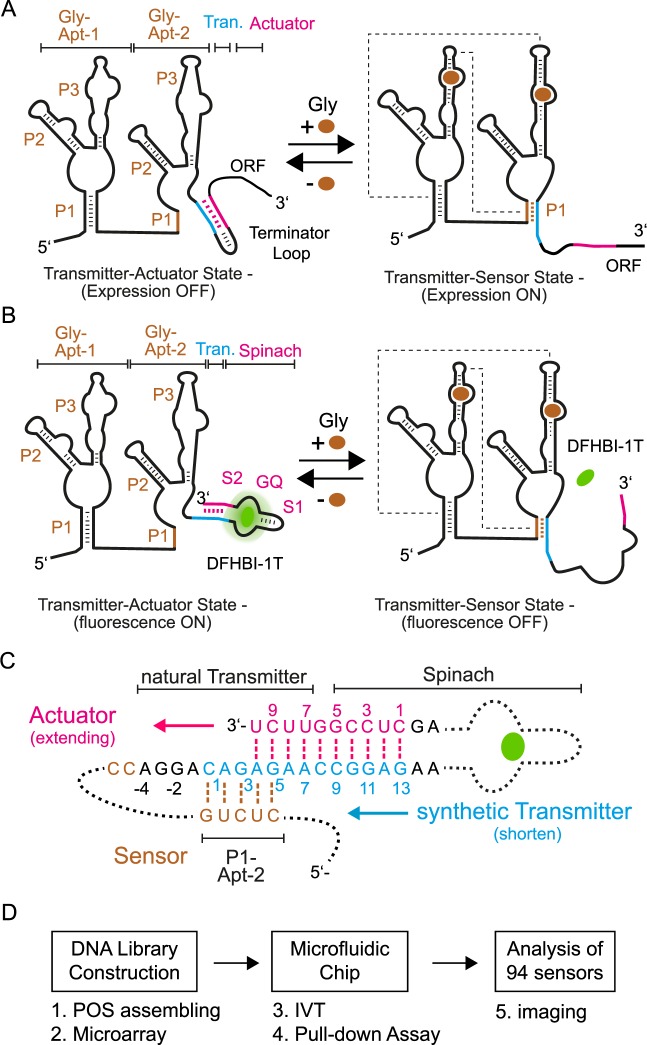
Figure 1.
Fluorogenic glycine riboswitch design and engineering approach. (A) Two state mechanism of the natural glycine riboswitch. In absence of glycine the transmitter (blue) hybridizes with the actuator (pink) and forms a transcription terminator loop, which decreases the expression of the following open reading frame (ORF). Upon binding of glycine to the sensor (brown), the two glycine aptamers dimerize. Dotted lines schematically indicate tertiary interactions between the two glycine aptamers. Consequently, the transmitter shifts its binding preference from the actuator strand toward the P1 stem of the Gly-Apt-2 and the transcription terminator loop is released. (B) Transfer of the natural two state folding mechanism of the glycine riboswitch to generate a fluorogenic readout signal. Here, glycine binding to the riboswitch shifts the transmitter binding preference from the Spinach actuator toward the P1 stem of the Gly-Apt-2. Thereby, the fluorogenic compound of Spinach (DFHBI-1T, green) is released. Stems of the two glycine aptamers (Gly-Apt-1 and 2) are labeled with P1-P3, whereas the stem-loop and G-quadruplex elements of the actuator are S1 and GQ, respectively. (C) The riboswitch library contained constructs with variable transmitter and counteracting actuator strands. The natural transmitter and stem sequences (S2) of Spinach are indicated. The nucleotide (nt) numbering starts for the transmitter sequence with the first nt overlap to the P1 stem of the sensor, whereas for the actuator strand it begins with the first complementary nt to the transmitter. (D) Workflow for functional screening of fluorogenic riboswitches with microfluidics (see also Supplementary Data).