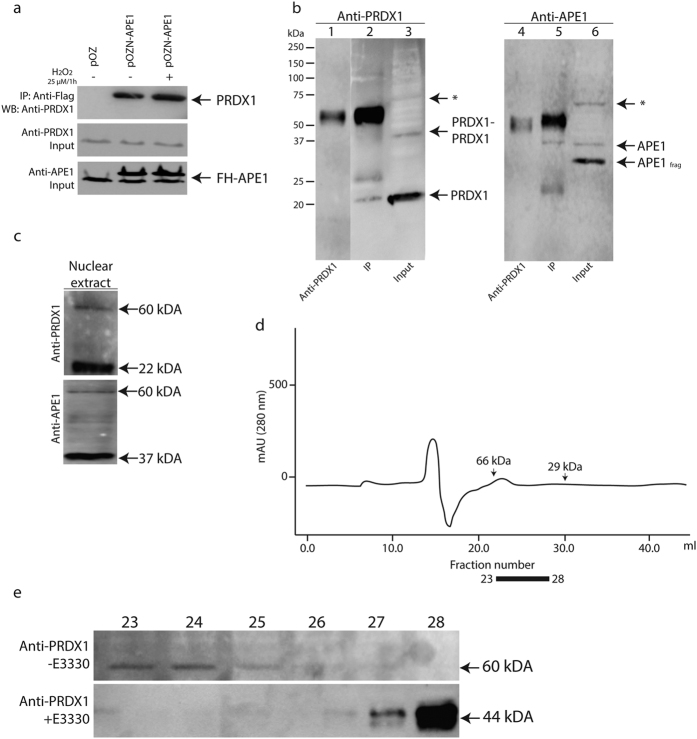
Figure 2. Validation of APE1-Prdx1 interaction.
(a) FH-APE1 pulled down Prdx1 during immunoprecipitation. HeLaS cells expressing FH-APE1 were untreated or treated with 25 μM H2O2 for 1 h, while the control HeLaS cells containing empty vector pOZ were untreated, and total cell extracts were subjected to immunoprecipitation with anti-FLAG resins. Eluates were separated by 10% SDS-PAGE and probed with monoclonal anti-PRDX1. (b) Reciprocal immunoprecipitation showing that PRDX1 pulls down APE1 from nuclear extract. Nuclear extract prepared from HeLa was subjected to immunoprecipitation with protein A magnetic beads bearing anti-PRDX1 antibodies. Lanes 2 and 5, immunoprecipitated proteins, lanes 3 and 6, the input nuclear extract, and lanes 1 and 4, each contained 0.5 μg of anti-PRDX1 antibodies alone. Following 10% SDS-PAGE for immunoblot analysis, lanes 1 to 3 were probed with anti-PRDX1 antibodies and lanes 4 to 6 with anti-APE1 antibodies. The arrows marked by an asterisk indicate a similar size polypeptide ~60 kDa detected by either anti-PRDX1 or anti-APE1 antibodies. (c) Detection of a 60 kDa polypeptide by either anti-PRDX1 or anti-APE1 antibodies in nuclear extract. Nuclear extract from HeLa cells prepared in the present of the thiol blocking agent methyl methanethiosulfonate (MMTS) was subjected to Western blot analysis probed with either anti-PRDX1 or anti-APE1 antibodies. (d) Analysis of APEX1-PRDX1 conjugate by fast protein liquid chromatography (FPLC). HeLa cells were untreated or treated with E3330 and the nuclear extract was loaded on superoseTM 6 FPLC column eluted with buffer A at flow rate of 0.25 ml/min. All eluents were collected at 0.5 ml/fraction, and each eluent fraction was concentrated by Amicon Ultra followed by Western blot analysis with 10% SDS PAGE. (e) The APE1 specific inhibitor E3330 prevents the appearance of the 60 kDa polypeptide and causes the accumulation of the dimeric PRDX1. Eluent fractions 23 to 28 from E3330 untreated and treated cells were probed with monoclonal anti-PRDX1. Fractions 23 and 24 contained the 60 kDa polypeptide from E3330 untreated cells, while fraction 28 contained the dimeric PRDX1 (44 kDa) from E3330 treated cells. The data are representative of two or three independent experiments.