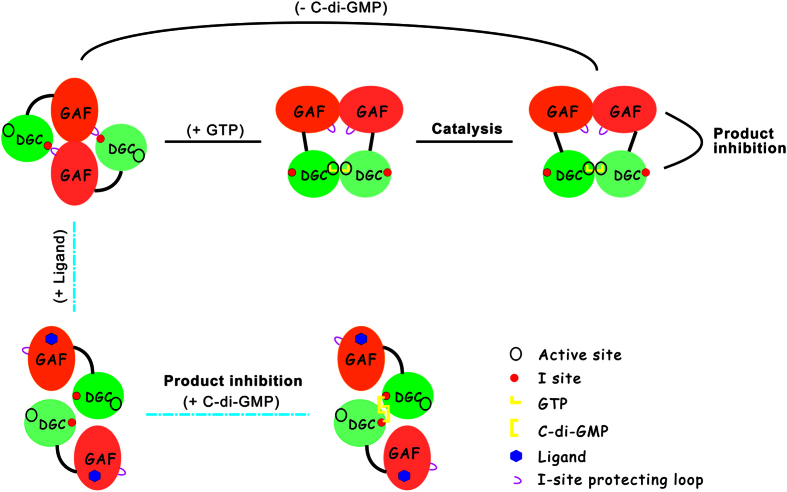
Figure 6. The proposed model for the activation and inhibition of Dcsbis.
The model demonstrates the potential regulation mechanism of Dcsbis activity. In the absence of the regulatory ligand, substrate GTP, and product c-di-GMP, the Dcisbis protein dimerizes via the GAF domain, with the I-site blocked by the protecting loop (represented by the full-length Dcsbis structure), and thus is highly active; Upon binding to GTP, the active sites of Dcsbis dimer get close to each other and catalyze the formation of c-di-GMP; c-di-GMP induces slight product inhibition. Binding of the potential regulatory ligand to the GAF domain may induce conformational change in the GAF domain, and thus break the GAF dimerization and lead to the release of the blockage of the I-site. At this condition, the product c-di-GMP could bind to the I-sites of GGDEF domain and bridge the formation of a new dimer (represented by the structure of GGDEF domain/c-di-GMP complex).