Abstract
Precise and accurate axon tract formation is an essential aspect of brain development. This is achieved by the migration of early outgrowing axons (pioneers) allowing later outgrowing axons (followers) to extend toward their targets in the embryo. In Caenorhabditis elegans the AVG neuron pioneers the right axon tract of the ventral nerve cord, the major longitudinal axon tract. AVG is essential for the guidance of follower axons and hence organization of the ventral nerve cord. In an enhancer screen for AVG axon guidance defects in a nid-1/Nidogen mutant background, we isolated an allele of aex-3. aex-3 mutant animals show highly penetrant AVG axon navigation defects. These defects are dependent on a mutation in nid-1/Nidogen, a basement membrane component. Our data suggest that AEX-3 activates RAB-3 in the context of AVG axon navigation. aex-3 genetically acts together with known players of vesicular exocytosis: unc-64/Syntaxin, unc-31/CAPS, and ida-1/IA-2. Furthermore our genetic interaction data suggest that AEX-3 and the UNC-6/Netrin receptor UNC-5 act in the same pathway, suggesting AEX-3 might regulate the trafficking and/or insertion of UNC-5 at the growth cone to mediate the proper guidance of the AVG axon.
Keywords: nervous system, axon guidance, pioneer, GEF, vesicle trafficking
PRECISE assembly of neuronal networks is a hallmark of a functional nervous system. Building these networks begins with early outgrowing axons from neurons called “pioneers.” Pioneer neurons form the initial axon scaffold used by the later outgrowing “follower” axons to extend upon. Pioneers provide guidance cues and an adhesive substrate for the follower axons to navigate properly. Sequential outgrowth of axons simplifies the problem of axonal pathfinding by allowing the majority of axons to extend along preexisting pathways rather than navigating exclusively on their own. Pioneer axons have been identified in many organisms. In grasshopper embryos, a pair of neurons (Ti) arise at the tips of the limb bud to extend axons toward the central nervous system. Follower (SGO) axons cannot extend further upon ablation of these pioneers (Klose and Bentley 1989). Similarly the Drosophila ventral nerve cord is pioneered by four axons forming longitudinal tracts. Ablation of these pioneers leads to defects in the navigation of follower axons (Hidalgo and Brand 1997). The thalamus of the mouse cerebral cortex is first invaded by short-lived subplate neurons, guiding later outgrowing cortical axons to their target (McConnell et al. 1989). However, pioneer neurons are not always required for the guidance of follower axons (Chitnis and Kuwada 1991) and in some cases are dispensable for this purpose (Keshishian and Bentley 1983; Eisen et al. 1989; Cornel and Holt 1992).
In Caenorhabditis elegans the major longitudinal axon tract is the ventral nerve cord (VNC) (White et al. 1986). It consists of two axon tracts flanking the ventral midline. The right axon tract contains most of the axons (∼50), whereas only around four axons form the left axon tract in the adult animal. The right side of the VNC harbors the main components of the motor circuit. This is where command interneurons connect to motor neurons, which in turn connect to nearby ventral muscles or more distant dorsal muscles. The corresponding synapses between interneurons and motor neurons can only be established between neurites in immediate contact. Even a local disorganization, i.e., axons in the “wrong neigborhood” will disrupt circuitry (White et al. 1976). Interneuron or motorneuron axons crossing into the left axon tract will not be able to establish the correct synaptic connections, unless their synaptic partners happen to join them.
Pioneers play an important role in creating this local organization within the C. elegans VNC (Durbin 1987; Garriga et al. 1993). The AVG neuron extends the first axon and pioneers the right VNC axon tract followed by motor neuron and interneuron axons in a defined order (Durbin 1987). Removal of the AVG axon early in development does not prevent the outgrowth of follower axons. The VNC still forms but is disorganized with axons crossing between right and left tracts (Durbin 1987; Hutter 2003). The left axon tract is pioneered by the PVPR axon from the posterior side (Durbin 1987). The left axon tract fails to form in the absence of the PVPR axon (Durbin 1987; Garriga et al. 1993), suggesting that no other neuron can pioneer this axon tract. Under such circumstances the follower axons extend in the already established right axon tract (Durbin 1987; Garriga et al. 1993).
Extracellular guidance cues also mediate outgrowth and navigation of axons and pioneer axons exclusively dependent on these guidance cues to navigate. UNC-6 (Netrin in vertebrates) is a laminin-like secreted protein that forms a gradient along the dorsoventral axis and is an essential cue for axons and cells migrating in a dorsoventral direction (Hedgecock et al. 1990; Ishii et al. 1992; Wadsworth et al. 1996; Wadsworth 2002). Cells and axons expressing the UNC-6 receptor UNC-40 (DCC in vertebrates) are attracted by UNC-6, whereas those expressing both UNC-40 and UNC-5 receptors are repelled, illustrating that response to a guidance cue can depend on receptor interactions within the neuron (Hedgecock et al. 1990; Ishii et al. 1992; Leung-Hagesteijn et al. 1992; Chan et al. 1996). UNC-129, a member of the TGF-β family, also affects dorsoventral migrations by promoting UNC-40 + UNC-5 signaling (Colavita et al. 1998; MacNeil et al. 2009). Dorsally expressed SLT-1/Slit repels axonal growth cones expressing the corrsponding SAX-3 receptors toward the ventral side (Zallen et al. 1998). Finally, NID-1/Nidogen, a basement membrane protein, is essential for correct positioning of axons in the sublateral nerve cord and VNC (Kim and Wadsworth 2000).
None of the known guidance cues substantially affects AVG axon navigation (Hutter 2003). Moreover, direct genetic screens for mutants affecting AVG navigation have yielded only a few new genes (Moffat et al. 2014; Bhat et al. 2015). This emphasized the need for other strategies like modifier screens to uncover AVG axon guidance genes. A good starting point for enhancer screens are nid-1 mutants, which have weakly penetrant AVG axon guidance defects, but are healthy and viable. We isolated an allele of aex-3 in an enhancer screen for AVG axon guidance defects in a nid-1 mutant background. aex-3 mutant animals display highly penetrant AVG axon guidance defects, which are dependent on nid-1. AEX-3, the homolog of the vertebrate MAP kinase-activating death domain (MADD) protein, is a guanine nucleotide exchange factor (GEF) for the Rab3 and Rab27 GTPases (Iwasaki et al. 1997; Mahoney et al. 2006), which control various aspects of vesicle trafficking in the cell (Wada et al. 1997; Hutagalung and Novick 2011; Zerial and McBride 2001). Our genetic interaction data suggest that AEX-3 activates Rab3, but not Rab27. We found aex-3 genetically interacts with UNC-64/Syntaxin, a SNARE component important for exocytosis of synaptic vesicles (Saifee et al. 1998) and dense core vesicles (Singer-Lahat et al. 2008). aex-3 also interacts with UNC-31/CAPS and IDA-1/IA-2, which are known to be involved in dense core vesicle release (Cai et al. 2004; Speese et al. 2007). In addition we found both UNC-6/Netrin and its receptor UNC-5 have nid-1-dependent AVG axon guidance defects. Genetic interaction data suggest aex-3 and unc-5 are in the same genetic pathway, suggesting that AEX-3 regulates the trafficking of the UNC-5 receptor to the growth cone and/or its insertion into the membrane at the growth cone.
Materials and Methods
Nematode strains and alleles used
The following strains were used for phenotypic analysis: VH1775: hdIs51[odr-2::tdTomato, rol-6(su1006)] X; VH1592: zdIs13[tph-1::GFP] IV; VH1811: hdIs54[flp-1::GFP,sra-6::plum, pha-1(+)]; VH15: rhIs4[glr-1::GFP,dpy-20(+)] III; VH612: hdIs24[unc-129::CFP, unc-47::DsRed2]; VH854: hdIs36[rgef-1::DsRed2]; and VH1762: leIs1722[W05H12.1::GFP, unc-119(+)]. UNC-5 overexpression strain is evIs98c [unc-5p::UNC-5::GFP] (Levy-Strumpf and Culotti 2014).
The following alleles were used for complementation: MT5475: aex-3(n2166) X; JT5: aex-3(sa5) X; and CX4103: sax-1(ky211) X.
The following alleles were used for phenotypic analysis and genetic interaction studies: VH2500: aex-3(hd148) X; MT5475: aex-3(n2166) X; JT5: aex-3(sa5) X; NM2739: aex-3(js815) X; NM791: rab-3(js49) II; JT24: aex-6(sa24) I; KY46: cab-1(tg49) X; CB169: unc-31(e169) IV; CB246: unc-64(e264) III; CB1265: unc-104(e1265) II; NM1657: unc-10(md1117) X; VH764: unc-6(ev400) X; VH10: unc-5(e53) IV; VH28: unc-40(e271) I; VC226: ida-1(ok409) III; and CH119: nid-1(cg119) V. The strains were cultured and maintained at 20° under standard conditions (Brenner 1974).
All our experimental strains generated from VH2500: aex-3(hd148); hdIs51, which harbors a closely linked point mutation in sax-1, may also contain the sax-1 mutation.
Isolation of the hd148 allele of aex-3
The hd148 allele of aex-3 was isolated after EMS mutagenesis of nid-1; hdIs51[odr-2::tdTomato,rol-6(su1006)] animals in a F2 semiclonal screen for axon guidance defects in the ventral cord pioneer AVG. Briefly, after EMS treatment, healthy P0s were allowed to self-propagate for two generations. F2 animals were then cloned and their progeny were analyzed under the dissecting fluorescence microscope for AVG axon cross-over defects leading to the identification of several mutants, including hd148. The hd148 mutant was backcrossed four times, and the whole genome was sequenced to identify changes in the coding regions of all genes. The hd148 mutation was linked to the X chromosome by crossing it with nid-1 males and then evaluating the phenotype of F1 males. aex-3 was picked as a potential candidate as the phenotypes (egg laying and body movement) of aex-3 matched with the isolated mutant. Complementation tests confirmed that hd148 is an allele of aex-3.
Fosmid rescue and expression constructs
The aex-3 containing fosmid (WRM0629dA08) was injected (1 ng/µl) into nid-1 mutant animals along with the coinjection marker myo-2::GFP (5 ng/µl) and filler DNA pBluescript KS (−) (94 ng/µl) to create transgenic lines. The nid-1 males with the hdEx602 [WRM0629dA08, (aex-3), myo-2::GFP] extrachromosomal array were then crossed with aex-3(hd148); nid-1 mutant animals, and the mutant animals with hdEx602 array were analyzed for the AVG axon cross-over defects. By injecting >600 adult animals we obtained only ∼60 F1’s and out of these, only one stable transgenic line, suggesting that the injected DNA might cause developmental problems. Other groups who worked with aex-3 encountered similar problems (Iwasaki et al. 1997; Iwasaki and Toyonaga 2000) .
To express aex-3 specifically in the AVG neuron, an enhancer fragment of lin-11, which drives expression specifically in the AVG neuron (B. Gupta, personal communication), and the aex-3 cDNA were cloned into GFP vector pPD95.75 (Fire vector kit). This construct (5–15 ng/µl) was injected along with coinjection marker unc-122::GFP (45 ng/µl) into nid-1 mutant animals. The nid-1 males with an extrachromosomal array were then crossed with aex-3(hd148); nid-1 mutant animals and the animals from three independent transgenic lines were analyzed for AVG axon cross-over defects. Transgenic lines for fosmid as well as expression vector were generated as described (Mello et al. 1991).
Construction of triple mutants
Triple mutants were constructed according to Iwasaki et al. (1997). The following primers were used to confirm the genotypes of strains containing the rab-3 (js49) and aex-3(hd148) mutants, both of which are point mutations not causing an unambigous movement defect like unc-31 and unc-64. The following forward 5′ CCAGCAGACAATACTTCGCC 3′ and reverse 5′ CTCCTTGGCTGATGTTCG 3′ primers were used to amplify a 610-bp PCR fragment from rab-3 genomic sequence including the change (G to A) in the rab-3(js49) mutant. Similarly the forward 5′ AGCACTTTTATACCCACTGG 3′ and reverse primer 5′ CTGGACAATGATGCTTTATTCAG 3′ primers were used to amplify a 529-bp PCR fragment from aex-3 genomic sequence including the change (G to A) in the aex-3(hd148) mutant.
Phenotypic analysis of neuronal defects
Axonal defects were scored with a Zeiss Axiscope (40× objective) in adult animals expressing fluorescent markers in respective neurons. Animals were immobilized with 10 mM sodium azide in M9 buffer for 1 hr and mounted on 3% agar pads before analysis.
Microscopy
Confocal images of mixed stage population of animals with respective fluorescent proteins were acquired on a Zeiss Axioplan II microscope (Carl Zeiss) connected to a Quorum WaveFX spinning disc system (Quorum Technologies). Stacks of confocal images with 0.2 to 0.5 μm distance between focal planes were recorded. Image acquisition and analysis was carried out using Volocity software (Perkin-Elmer, Waltham, MA). Images in the figures are maximum intensity projections of all focal planes. Figures were assembled with Adobe Photoshop CS8.0 (Adobe, San Jose, CA).
Statistical analysis
We used chi-square tests (χ2) to determine the statistical significance of differences in phenotypes. Multiple comparisons were corrected by a Bonferroni correction, where the given alpha value (α) was divided by the number of comparisons (n) made so that for statistical significance in multiple comparisons individual P values are ≤α/n.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results
Identification of a novel allele of aex-3
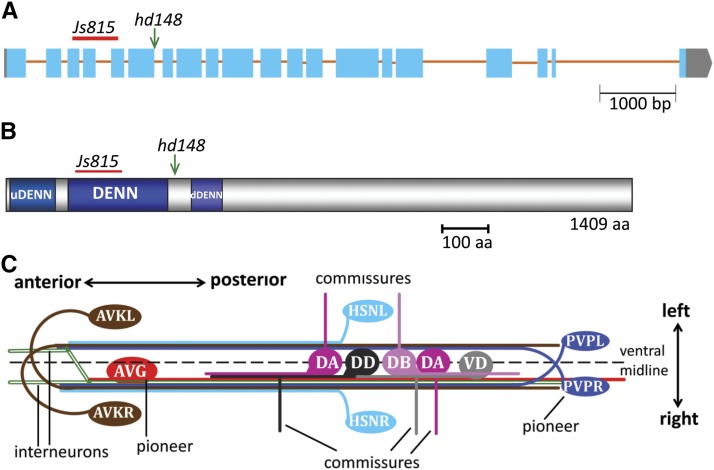
The molecular basis for AVG axon guidance is only partially understood. We performed enhancer screens in a nid-1 mutant background to identify novel regulators of AVG navigation. We used the deletion allele nid-1(cg119), which deletes 3133 nucleotides from 1499 to 4631 and is considered a molecular null of NID-1 (Kang and Kramer 2000). One of the alleles we isolated, hd148, is a point mutation (G to A) in the splice donor at the beginning of the 6th intron of aex-3, preventing the removal of this intron and resulting in the early truncation of the protein after 388 of 1409 amino acids (Figure 1, A and B). Previously isolated aex-3 mutant animals have some locomotion and egg laying defects (Thomas 1990; Iwasaki et al. 1997; Mahoney et al. 2006) similar to defects seen in hd148. Three known alleles of aex-3: n2166, sa5, and js815 (Thomas 1990; Mahoney et al. 2006), showed AVG axon cross-over defects in a nid-1 mutant background (Table 1). Two of the alleles, n2166 and sa5, failed to complement hd148 for the AVG navigation defects. Complementation tests were done so that half of the progeny were hd148/n2166; nid-1/nid-1 and the remaining half hd148/n2166; nid-1/+. This mixture showed 31% AVG cross-over defects (n = 70) and similarly the mixture of hd148/sa5; nid-1/nid-1 and hd148/sa5; nid-1/+ F1 animals showed 32% AVG cross-over defects (n = 84). Since the AVG cross-over defects of hd148 mutants are strictly dependent on the nid-1 mutation, these defects are not different from 56% AVG cross-over defects shown by our hd148 alone, if the 50% nid-1 heterozygote progeny are ignored, indicating that hd148 does not complement known alleles of aex-3. As revealed by whole genome sequencing the hd148-containing strain also carries a misssense mutation in sax-1, a gene closely linked to aex-3. sax-1 is required for neurite initiation and outgrowth in sensory neurons (Zallen et al. 2000). However, sax-1(ky211) complements hd148 for AVG navigation defects as the mixture of hd148/ky211; nid-1/nid-1 and hd148/ky211;nid-1/+ F1 animals showed only 10% AVG cross-over defects (n = 62), suggesting that the mutation in sax-1 does not contribute to the navigation defects seen in our isolate. In addition, a fosmid containing the aex-3 gene rescued the AVG axon cross-over defects of aex-3(hd148); nid-1(cg119) mutant animals. The rescuing line shows only 24% AVG axon cross-over defects, significantly fewer than the 56% defects seen in aex-3; nid-1 mutant animals (Table 2). Taken together, these data suggest that hd148 is an allele of aex-3.
Figure 1.
Molecular analysis of aex-3 and schematics of the VNC. (A) Gene model for aex-3 with exons shown as light blue shaded boxes and introns as dark brown lines. The gray shaded boxes in the beginning and end represent the 5′ and 3′ untranslated regions, respectively. The location for the hd148 and js815 alleles is also shown. hd148 is a point mutation in the beginning of intron 6, which affects splicing and leads the formation of the stop codon. js815 is a 555-bp deletion removing 159–264 aa (Mahoney et al. 2006), which changes the reading frame and leads the formation of the stop codon. (B) Domain organization of the AEX-3 protein. The positions of mutations are also indicated. (C) Schematic showing different types of neurons present in the VNC. The position of cell bodies and neuronal processes are also shown. Left–right and anterior–posterior sides are as indicated. For simplicity, only some of the motor neurons are shown.
Table 1. AVG cross-over (CO) defects in aex-3 mutants with and without nid-1 (% animals with defects).
| Genotype | AVG CO | n |
|---|---|---|
| aex-3(hd148) | 4* | 104 |
| aex-3(hd148);nid-1 | 56** | 97 |
| aex-3(n2166) | 3 ns | 74 |
| aex-3(n2166);nid-1 | 46** | 112 |
| aex-3(sa5) | 3 ns | 95 |
| aex-3(sa5);nid-1 | 43** | 130 |
| aex-3(js815) | 4* | 121 |
| aex-3(js815);nid-1 | 39** | 112 |
| nid-1(cg119) | 10** | 118 |
| Wild type | 0 | 117 |
Marker used: hdIs51[odr-2::tdTomato]. n = number of animals. For statistical significance, single mutants were compared with wild type and double mutants with nid-1 single mutant. * P < 0.05; ** P < 0.01; ns, not significant; χ2 test.
Table 2. AVG cross-over defects are rescued by an aex-3-containing fosmid, expression of the cDNA in AVG and unc-5 overexpression (% animals with defects).
| Genotype | AVG CO | n |
|---|---|---|
| aex-3 fosmid (WRM0629Da08) | ||
| aex-3(hd148); nid-1; hdEx602(+) | 24** | 102 |
| aex-3(hd148); nid-1; hdEx602(−) | 50 ns | 104 |
| aex-3 cDNA | ||
| aex-3(hd148); nid-1; hdEx610(+) | 18** | 128 |
| aex-3(hd148); nid-1; hdEx610(−) | 45 ns | 95 |
| aex-3(hd148); nid-1; hdEx611(+) | 16** | 130 |
| aex-3(hd148); nid-1; hdEx611(−) | 48 ns | 100 |
| aex-3(hd148);nid-1; hdEx612(+) | 13** | 116 |
| aex-3(hd148); nid-1; hdEx612(−) | 47 ns | 94 |
| unc-5 overexpression | ||
| aex-3(hd148); nid-1; evIs98c | 25** | 132 |
Marker used: hdIs51[odr-2::tdTomato]. n = number of animals. For rescue experiments, animals with (+) and without (−) extrachromosomal arrays were counted. For the aex-3 rescue the following independent transgenic lines were analyzed: hdEx602 [WRM0629dA08; myo-2::GFP]; hdEx610, 611, and 612 [plin-11::aex-3 cDNA::GFP; unc-122]; and evIs98c [unc-5p::UNC-5::GFP]. For statistical significance, we compared transgenic lines with aex-3(hd148); nid-1 double mutant. ** P < 0.01; ns, not significant; χ2 test.
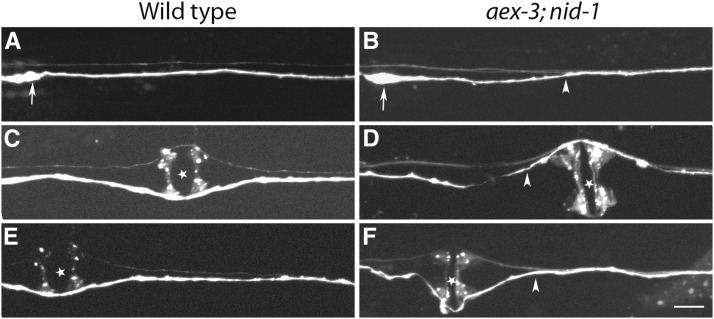
aex-3 mutant animals show nid-1-dependent AVG axon navigation defects
In wild-type animals the AVG axon pioneers the right axon tract of the VNC extending from the anterior end and terminating in the tail (Figure 1C and Figure 2, A, C, and E). In 56% of aex-3(hd148); nid-1 mutant animals the AVG axon crosses the ventral midline from right to left, referred to as AVG cross-over (AVG CO) defects. These cross-overs can occur anywhere along the anterior–posterior axis (Figure 2, B, D, and F), but occur more frequently in the anterior half of the animal. After crossing, AVG axons remain in the left axon tract in the majority of animals. However, in 19% of the mutant animals, AVG axons cross back to the right axon tract. AVG cross-over defects in aex-3 are almost completely dependent on nid-1, as aex-3(hd148) on its own has only 4% defects (Table 1), whereas nid-1 single mutant animals have ∼10% AVG cross-over defects (Table 1). Three other alleles (n2166, sa5, and js815) of aex-3 show nid-1-dependent AVG cross-over defects with a penetrance similar to hd148 (Table 1). Defects are somewhat higher in hd148, but not significantly different from the other alleles except for js815, which has the least penetrant defects (Table 1). The molecular nature of the hd148 allele—expected to lead to an early truncation of the protein—matches well with the strong defects observed and suggests it is a strong loss-of-function allele.
Figure 2.
AVG cross-over (CO) defects in aex-3(hd148); nid-1 mutant animals. (A, C, and E) wild type; (B, D, and F) aex-3(hd148); nid-1. (A, C, and E) In wild-type animals, AVG extends an axon from its cell body (arrow) on the right side of the VNC that never crosses the ventral midline. In the majority of the aex-3(hd148); nid-1 mutant animals the AVG axon crosses the ventral midline somewhere in the anterior half (B and D) and in some animals in the posterior half of the animal (F) and joins the left axon tract of the VNC. The AVG axon stays mostly on the left side of the VNC after crossing the midline but in some animals it switches back to the right side. Arrows indicate the position of the AVG cell body, arrowheads indicate the position of AVG axon cross-over ,and the stars mark the vulva position. Marker used: odr-2::tdTomato. Bar: 10 µm.
Follower axon navigation is disrupted in aex-3; nid-1 mutant animals
Since the AVG pioneer is important for correct organization of the VNC, we wanted to test whether follower axons are also affected, and we evaluated aex-3(hd148); nid-1(cg119) mutant animals with neuron-specific markers for follower defects. Command interneurons initially exit the nerve ring in two fascicles, one each on the right and left sides. Immediately after leaving the nerve ring, the left side fascicle crosses and extends into the right axon tract and both fascicles follow the AVG axon (Figure 1C and Figure 3E). In aex-3; nid-1 double mutant animals, 49% of animals show cross-over defects where interneuron axons cross from the right to the left side of the VNC (Figure 3F; Table 3). Among these, 35% are AVG dependent, i.e., interneurons cross the midline at the same position where the AVG axon crosses (Figure 3F; Table 3). In the remaining 14% of animals, interneuron axons cross independently of the AVG axon (Table 3). This suggests that while the majority of the interneuron cross-over defects are likely secondary consequences of AVG defects, some of the defects are likely primary defects in interneurons themselves.
Figure 3.
Interneuron, AVK, and HSN axon defects in aex-3(hd148); nid-1 mutant animals. (A, C, and E) Wild type; (B, D, F, F′, and F′′) aex-3(hd148); nid-1. (A) In wild-type animals, AVK axons extend in both VNC axon tracts and never cross the midline. (B) In aex-3(hd148); nid-1 mutant animals, AVK axons frequently cross the ventral midline (arrowhead). (C) In wild-type animals, HSN axons are ipsilateral and extend axons in both VNC axon tracts on their respective sides. (D) In the majority of the aex-3(hd148); nid-1 mutant animals, the left HSN axon crosses the ventral midline and joins the right axon tract (arrowhead). (E) In wild-type animals, command interneurons extend into the right axon tract of the VNC. (F and F′) In aex-3(hd148); nid-1 mutant animals, the command interneurons cross the midline frequently together with the AVG axon and extend into the left axon tract of the VNC. F′′ is the merged image of F and F′. Stars mark the vulva position. The dashed lines (white) mark the normal axon trajectories. Markers used: glr-1::GFP (interneurons), flp-1::GFP (AVK), and tph-1:: GFP (HSN). Bar: 10 µm.
Table 3. Other neuronal defects in nid-1 single and aex-3(hd148); nid-1 double mutants (% animals with defects).
| Phenotype | aex-3(hd148) | nid-1(cg119) | aex-3(hd148); nid-1 |
|---|---|---|---|
| Interneuron cross-over defects | |||
| AVG dependenta | 6 (101) | 6 (114) | 35** (104) |
| AVG independentb | 5 (101) | 0 (114) | 14** (104) |
| AVK defects | |||
| VNC cross-over | 6 (107) | 18 (115) | 49** (109) |
| Premature stop | 0 (107) | 12 (115) | 22 ns (109) |
| Leaving VNC | 0 (107) | 1 (115) | 3 ns (109) |
| HSN neurons | |||
| VNC cross-over | 9 (132) | 58 (103) | 68 ns (109) |
| Undermigration and VNC cross-over | 6 (132) | 3 (103) | 16** (109) |
| Motor neuron defects | |||
| DD/VD | |||
| VNC cross-overc | 6 (102) | 8 (133) | 28** (123) |
| Symmetrical VNC | 0 (102) | 0 (133) | 36** (123) |
| Commissural defects | 14 (102) | 65 (133)d | 100** (123)e |
| DNC gaps | 31 (95) | 85 (53) | 100* (26) |
| DA/DB | |||
| VNC cross-overf | 6 (101) | 8 (110) | 29** (103) |
| Symmetrical VNC | 0 (101) | 0 (110) | 20** (103) |
| Commissural defects | 0 (101) | 0 (110) | 18** (103) |
Values in parentheses indicate n. Markers used: Interneuron (glr-1::GFP), AVK (flp-1::GFP), HSN (tph-1::GFP), DD/VD/Commissures (unc-47::DsRed-2), and DA/DB (unc-129::CFP). For statistical significance, aex-3; nid-1 double mutants are compared with nid-1 single mutant. * P < 0.05; ** P < 0.01; ns, not significant; χ2 test.
Interneurons cross the midline together with AVG.
Interneurons cross the midline without AVG.
DD/VD axons cross the midline at the same position where the AVG axon crosses.
On average, 5 of 19 commissures fail to reach the DNC per animal.
On average, 7 of 19 commissures fail to reach the DNC per animal.
DA/DB axons cross the midline at the same position where the AVG axon crosses.
The two AVK neurons also send axons into the VNC from the nerve ring. However in this case one of them extends in the left tract, whereas the other one extends in the right tract (Figure 1C and Figure 3A). In aex-3; nid-1 double mutant animals we observed that both AVKR/L axons frequently cross the ventral midline with equal penetrance (Figure 3B; Table 3). However we did not observe any correlation between the AVG and AVK axon cross-overs, suggesting that these defects are likely independent. In some mutant animals AVK axons terminate prematurely, but this is not significantly different from nid-1 single mutants (Table 3).
The hermaphrodite-specific neurons (HSN) neurons are a symmetrical pair of neurons born in the tail during embryogenesis. They migrate toward the midbody, where each neuron extends an axon on either side of the ventral midline along the VNC toward the nerve ring (Figure 1C and Figure 3C). nid-1 single mutants have highly penetrant HSN cross-over defects, which are not enhanced in aex-3; nid-1 double mutant animals (Figure 3D; Table 3). However in aex-3; nid-1 double mutant animals some HSN neurons fail to migrate properly, defects not seen in nid-1 single mutants (Table 3).
aex-3; nid-1 mutant animals show motor neuron axon navigation defects
The VNC in C. elegans contains essential components of the motor circuit. DD/VD GABAergic motor neuron cell bodies are located along the ventral midline and send out processes in the right axon tract that branch and extend commissures dorsally (Figure 1C and Figure 4, A, C, and E). In aex-3; nid-1 double mutants, 28% of animals show DD/VD axon guidance defects, where motor neuron axons cross from the right to the left tract of the VNC (Figure 4B; Table 3). These defects are mostly dependent on AVG, since DD/VD axons cross together with the AVG axon. Additionally in 36% of aex-3; nid-1 mutant animals, some DD/VD axons grow in the left rather than the right axon tract, which makes the VNC symmetrical in appearance (symmetric VNC) (Figure 4, B and D; Table 3). DD/VD commissures individually (as pioneers) navigate toward the dorsal side, where they extend to form the dorsal nerve cord (DNC) (Figure 4, E and G). A total of 65% of nid-1 single mutants have commissural defects, where on average, 5 of 19 commissures per animal fail to reach the dorsal cord leading to gaps (DNC gaps, Table 3). In aex-3 single mutants, 14% of animals have commissural defects (1 of 19 commissures fails to reach the dorsal side, i.e., <1% of commissures show defects) with fewer gaps in the DNC (Table 3). We observed that in aex-3; nid-1 double mutant animals these defects are enhanced to 100%, with every animal showing commissural defects (7 of 19 commissures per animal fail to reach the DNC) and gaps in the DNC (Figure 4, F and H; Table 3). This suggests that aex-3 is required independently of nid-1 for the guidance of commissures and the proper formation of the DNC.
Figure 4.
DD/VD and DA/DB motor neuron defects in aex-3(hd148); nid-1 mutant animals. (A, C, E, G, I, K, and M) Wild type; (B, D, F, H, J, L, and N) aex-3(hd148); nid-1. (A, C, E, and G) In wild-type animals, DD/VD motor neurons extend axons anteriorly in the right axon tract that branch and extend commissures toward the dorsal side to form the DNC. In aex-3(hd148); nid-1 mutant animals motor neuron axons either cross the ventral midline (arrowhead) and extend into the left axon tract (B) or grow directly into the left axon tract (arrow) (B and D). Some DD/VD commissures (F) fail to reach the dorsal cord, resulting in gaps (H, arrow with two heads). (I, K, and M) In wild-type animals, DA/DB motor neurons extend dendrites into the right axon tract and commissures that grow from the cell body toward the dorsal cord. (J, L, and N) In aex-3(hd148); nid-1 mutant animals, DA/DB dendrites cross the ventral midline (arrowhead) and extend in the left axon tract (J) or grow directly in the left axon tract (L, arrow). Some commissures show navigation defects (N). Stars mark the vulva position. Markers used: unc-47::DsRed2 (DD/VD), unc-129::CFP (DA/DB). Bar: 10 µm.
DA/DB motor neuron cell bodies are also located along the ventral midline. They send their dendrites into the right axon tract of the VNC and axons toward the DNC (Figure 1C and Figure 4, I, K, and M). We found in 29% of aex-3; nid-1 double mutant animals, DA/DB motor axons cross from the right to the left axon tract of the VNC (Figure 4J; Table 3). These defects are mostly dependent on AVG, as cross-overs occur together with the AVG axon, again suggesting these are secondary defects. In addition, some DA/DB motor neurons extend dendrites into the left axon tract (Figure 4L; Table 3). We observed very few DA/DB commissural defects (Figure 4N) and no DNC gaps in aex-3; nid-1 double mutant animals, suggesting commisural defects are neuron specific.
We used a panneuronal marker to assess the overall state of the nervous system as well as selected neurons with axons outside the VNC. We did not observe guidance defects in touch receptor axons, other longitudinal axons, or neuronal processes in the head region (data not shown). Defects in aex-3; nid-1 mutant animals are largely confined to the VNC and some commissural axons.
AEX-3 activation of RAB-3 GTPase is required for AVG navigation
AEX-3 is a GEF and regulates the activity of the RAB-3 and AEX-6/Rab27 GTPases (Mahoney et al. 2006). Both bind to synaptic vesicle precursors in their GTP form and regulate trafficking of these vesicles (Nonet et al. 1997; Mahoney et al. 2006). In wild type, RAB-3 and AEX-6/Rab27 are localized to axons and enriched in synaptic regions, whereas in aex-3 mutant animals, RAB-3 as well as AEX-6/Rab27 are mislocalized to the cell body (Iwasaki and Toyonaga 2000; Mahoney et al. 2006). We found that mutations in nid-1 do not affect RAB-3 localization. Even though both RAB-3 and AEX-6/Rab27 are activated by AEX-3, they act through different downstream effectors (Mahoney et al. 2006). To test whether AEX-3 regulates RAB-3 or AEX-6/Rab27 (or both) in the context of AVG axon navigation, we constructed rab-3; nid-1 and aex-6; nid-1 double mutants and evaluated them for AVG axon cross-over defects. rab-3; nid-1 mutant animals show AVG axon cross-over defects with a penetrance similar to aex-3; nid-1, whereas aex-6; nid-1 double mutants do not (Figure 5). rab-3 single mutants have weakly penetrant AVG axon cross-over defects and aex-6 single mutants do not have any such defects (Figure 5). The penetrance of AVG axon cross-over defects in aex-3; rab-3; nid-1 triple mutant animals is not significantly different from aex-3; nid-1 double mutants (Figure 5), suggesting that aex-3 and rab-3 are in the same genetic pathway. This indicates that activation of RAB-3 but not AEX-6/Rab27 is required for AVG navigation.
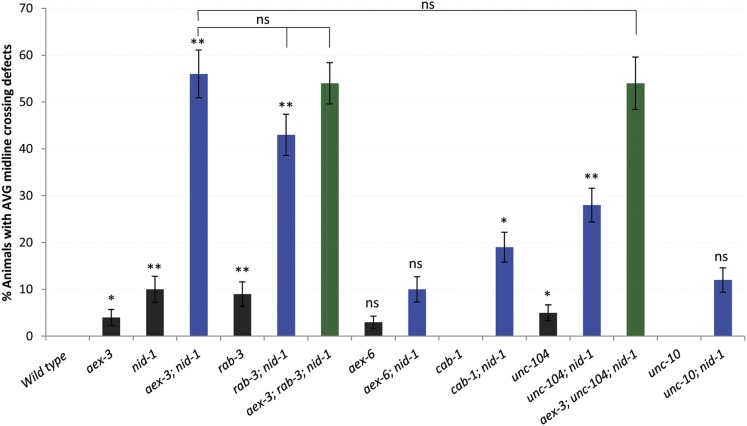
Figure 5.
aex-3 and rab-3 act in the same genetic pathway in AVG axon navigation. Columns are percentages of animals with AVG midline crossing defects (±SE) with genotypes as indicated. In the bar diagram, the single mutants are represented by black bars, the double mutants by blue bars, and the triple mutants by green bars. For each strain, n > 100 animals except for aex-3; nid-1 (n = 97). χ2 tests were used to establish statistical significance between the mutants. Single mutants were compared with wild type, double mutants with nid-1 single mutant, and the triple mutants with aex-3; nid-1 double mutant (* P < 0.05; ** P < 0.01; ns, not significant). Mutant alleles used: aex-3(hd148), nid-1(cg119), rab-3(js49), aex-6(sa24), cab-1(tg49), unc-104(e1265), and unc-10(md1117). Marker used: odr-2::tdTomato.
AEX-3 physically interacts with CAB-1 to regulate defecation, a pathway distinct from the rab-3 pathway (Iwasaki and Toyonaga 2000). We tested cab-1; nid-1 double mutant animals for AVG axon cross-over defects and observed only a mild enhancement of the defects (Figure 5), suggesting CAB-1 does not have a major role in the context of AVG navigation.
Since RAB-3-associated vesicles are transported by the UNC-104/Kinesin-3 motor in C. elegans (Hall and Hedgecock 1991; Nonet et al. 1997), we wanted to test whether UNC-104 plays any role here. AVG axon cross-over defects are enhanced in unc-104; nid-1 double mutant animals compared to either single mutant, but these defects are less penetrant compared to aex-3; nid-1 or rab-3; nid-1 double mutants (Figure 5). However, the penetrance of AVG cross-over defects in aex-3; unc-104; nid-1 triple mutants is not significantly different from the aex-3; nid-1 double mutants (Figure 5). This suggests that aex-3 and unc-104 are in the same genetic pathway and that AEX-3 could be involved in transport of vesicles in outgrowing axons in part mediated through UNC-104.
UNC-10/Rim is a RAB-3 effector molecule, which interacts with RAB-3 during priming of synaptic vesicles prior to their release at the synapse (Koushika et al. 2001). unc-10 single mutants have no AVG axon cross-over defects, and nid-1 defects are not enhanced in unc-10; nid-1 double mutants (Figure 5), indicating that UNC-10/Rim is not involved in the navigation of the AVG axon. This is not unexpected as there are no synapses at that stage of pioneer axon outgrowth. In summary, the above data suggest that AEX-3 activates RAB-3 but not AEX-6/Rab27 in the context of AVG navigation and is potentially involved in UNC-104-dependent vesicle transport.
Given that AEX-3 regulates RAB-3 in the context of AVG navigation, one would expect AEX-3 to act in a cell-autonomous manner. To determine whether AEX-3 is required within the AVG neuron for its axon navigation, we expressed an AEX-3 complementary DNA (cDNA) specifically in the AVG neuron in aex-3; nid-1 double mutants. We evaluated the AVG axon cross-over defects in these transgenic lines and observed that AEX-3 was able to rescue the AVG axon cross-over defects in all three independent transgenic lines (Table 2). For all three lines, both animals with and without extrachromosomal arrays were counted. Defects were only rescued in animals containing the extrachromosomal array (Table 2). Thus AEX-3 is required in the AVG neuron for its axon navigation and acts in a cell-autonomous manner.
AEX-3, UNC-31/CAPS, IDA-1/IA-2, and UNC-64/Syntaxin act in the same genetic pathway
aex-3 genetically interacts with unc-31 and unc-64 in dauer formation constitutive (Daf-c) phenotype (Iwasaki et al. 1997). UNC-31 is the nematode homolog of CAPS (Ca2+-dependent secretion activator) and is required for secretion of dense core vesicles (Livingstone 1991; Walent et al. 1992; Speese et al. 2007). unc-64 encodes the nematode homolog of Syntaxin, a SNARE component involved in the fusion of synaptic vesicles (Saifee et al. 1998). Recently it has been shown that Syntaxin1 binds to the guidance receptor DCC in the growth cone, where it is required for chemoattraction of migrating axons to the guidance cue Netrin (Cotrufo et al. 2012). We wanted to test whether unc-31 and unc-64 mutants have AVG axon guidance defects similar to those seen in aex-3. Neither unc-31 nor unc-64 single mutants have AVG axon cross-over defects; however, both mutants enhance AVG defects of nid-1 mutants but do not enhance aex-3 mutant defects (Figure 6). Both unc-31; nid-1 and unc-64; nid-1 double mutants have defects that are significantly less penetrant than defects in aex-3; nid-1 double mutants (Figure 6). However, both unc-31; aex-3; nid-1 and unc-64; aex-3; nid-1 triple mutants are not significantly different from defects in aex-3; nid-1 double mutants (Figure 6), suggesting that all three genes act in the same pathway.
Figure 6.
aex-3, ida-1, unc-31, and unc-64 act through the same genetic pathway in AVG axon navigation. Columns are percentages of animals with AVG midline crossing defects (±SE) with genotypes as indicated. In the bar diagram, the single mutants are represented by black bars, the double mutants by blue bars, and the triple mutants by green bars. For each strain, n > 100 except aex-3; nid-1 (n = 97) animals. χ2 tests were used to establish statistical significance between the mutants. Single mutants were compared with wild type, double mutants with the strongest single mutant, and the triple mutants with aex-3; nid-1 double mutant (* P < 0.05; ** P < 0.01; ns, not significant). Mutant alleles used: aex-3(hd148), nid-1(cg119), ida-1(ok409), unc-31(e169), and unc-64(e264). Marker used: odr-2::tdTomato.
unc-31 has been shown to interact with ida-1 for dense core vesicle exocytosis (Cai et al. 2004). ida-1; nid-1 double mutant animals have AVG axon cross-over defects with a penetrance close to aex-3; nid-1 double mutants, whereas aex-3; ida-1 double mutants do not show these defects (Figure 6). Defects in ida-1; aex-3; nid-1 triple mutant are not enhanced compared to aex-3; nid-1 double mutants (Figure 6), again suggesting that these genes act in the same pathway.
AEX-3 might act through the Netrin receptor UNC-5
Since Syntaxin1 (UNC-64) has recently been directly implicated in Netrin-mediated axonal navigation (Cotrufo et al. 2012), we examined whether UNC-6/Netrin is involved in AVG axon navigation. unc-6 mutant animals display only weakly penetrant AVG axon cross-over defects similar to nid-1 mutant animals (Figure 7). AVG defects are significantly enhanced in unc-6; nid-1 double mutants, however not as much as in aex-3; nid-1 double mutants (Figure 7). This suggests unc-6 indeed plays a role in the navigation of the AVG axon. Two receptors, UNC-40/DCC and UNC-5, mediate responses to UNC-6/Netrin in C. elegans. unc-5 mutants have mild AVG cross-over defects, but unc-40 mutants do not and unc-5 defects are not enhanced in aex-3; unc-5 double mutants (Figure 7). unc-5; nid-1 double mutant animals have penetrant AVG cross-over defects comparable to aex-3; nid-1 (Figure 7). These defects are not further enhanced in unc-5; aex-3; nid-1 triple mutants. In contrast, unc-40 does not enhance nid-1 defects (Figure 7). This suggests UNC-5, but not UNC-40, is required for the navigation of the AVG axon.
Figure 7.
aex-3 and unc-5 act through the same genetic pathway in AVG axon navigation. Columns are percentages of animals with AVG midline crossing defects (±SE) with genotypes as indicated. In the bar diagram, the single mutants are represented by black bars, the double mutants by blue bars, and the triple mutant by a green bar. For each strain, n > 100 except aex-3; nid-1 (n = 97) animals. χ2 tests were used to establish statistical significance between the mutants. Single mutants were compared with wild type, double mutants with the strongest single mutant, and the triple mutant with aex-3; nid-1 double mutant (* P < 0.05; ** P < 0.01; ns, not significant). Mutant alleles used: aex-3(hd148), nid-1(cg119), unc-6(ev400), unc-40(e271), and unc-5(e53). Marker used: odr-2::tdTomato.
The above data suggest that AEX-3 might regulate trafficking of the UNC-5 receptor to the AVG growth cone or insertion of the receptor into the cell membrane of the growth cone. Since aex-3 mutants do not show an unc-5 loss-of-function phenotype, aex-3 cannot be absolutely required for transport of UNC-5 but it could reduce the effectiveness of the transport such that navigation defects become apparent only in a sensitized (i.e., nid-1 mutant) background. We found that a functional UNC-5::GFP overexpression construct (Levy-Strumpf and Culotti 2014) partially rescues the AVG cross-over defects of aex-3; nid-1 mutant animals (Table 2). We then used the same UNC-5::GFP reporter to determine whether there are obvious changes in UNC-5::GFP levels in growth cones in aex-3 single and aex-3; nid-1 double mutant animals. While it is not technically possible to visualize the UNC-5::GFP reporter in the AVG growth cone, it can be observed in postembryonic VD growth cones as they navigate toward the dorsal cord. UNC-5::GFP expression in VD growth cones of both aex-3 single and aex-3; nid-1 double mutant animals is comparable to expression in wild type (Figure 8), suggesting that as expected, aex-3 is not absolutely essential for transport of UNC-5 to the growth cone. Since GFP expression levels and GFP distribution in individual growth cones are quite variable, we found it difficult to determine whether there are more subtle changes in UNC-5::GFP expression levels. Furthermore the UNC-5::GFP reporter is mainly localized in puncta (likely vesicles) within the growth cone and not localized to the plasma membrane as expected and as observed with an UNC-40::GFP reporter (Norris et al. 2014). It is therefore impossible to judge with this reporter whether insertion of UNC-5::GFP into the plasma membrane of growth cones is affected in aex-3 mutant animals. Taken together the observations support a role for aex-3 in trafficking of UNC-5.
Figure 8.
Localization of UNC-5::GFP in growth cones in aex-3 mutants. Fluorescent micrographs of postembryonic VD growth cones of L2 stage larvae are shown. In wild type, aex-3 single and aex-3; nid-1 double mutant animals, UNC-5::GFP is localized to the growth cones as indicated by white arrows. White star highlights fluorescent granules in the gut. Bar: 5 µm. Marker strain used: evIs98c [unc-5p::UNC-5::GFP].
Discussion
Ventral cord axon guidance requires aex-3 in a Nidogen-dependent manner
The AVG axon pioneers the right axon tract of the VNC in C. elegans and is critical for the navigation of follower axons (Durbin 1987; Hutter 2003). We isolated an allele of aex-3 in an enhancer screen for AVG axon guidance defects in a nid-1 mutant background. In C. elegans, nid-1 encodes the sole homolog of nidogen (entactin), a basement membrane component. nid-1 is required for the correct positioning of longitudinal axons and proper organization of presynaptic zones (Kim and Wadsworth 2000; Ackley et al. 2003), but not for basement membrane assembly in general. nid-1 mutant animals thus are viable and healthy. nid-1 mutant animals have weakly penetrant AVG axon cross-over defects, which are substantially enhanced in aex-3; nid-1 double mutants, suggesting that aex-3 is required for AVG axon navigation. AEX-3 is the nematode homolog of the GDP/GTP exchange factor (GEF) for Rab3/Rab27, and is expressed in most neurons (Iwasaki et al. 1997). AEX-3 activates the RAB-3 and AEX-6/Rab27 GTPases through distinct pathways regulating synaptic vesicle transport and exocytosis (Mahoney et al. 2006). Since aex-3 and nid-1 are not obviously in a common pathway, synergistic effects on axon pathfinding are likely due to the disruption of functionally redundant pathways in nid-1 mutants. How nid-1 controls axon navigation is currently unknown.
AVG pioneer navigation defects were expected to have secondary consequences on follower axon navigation (Hutter 2003). We found navigation defects in several classes of VNC follower neurons in aex-3; nid-1 mutant animals. In many cases these axons followed misguided pioneers, suggesting these defects are indeed secondary. However, in some cases follower defects were not correlated with pioneer defects, raising the possibility that primary defects are not limited to AVG. For example, we found no correlation between AVK and AVG defects, indicating that AVK defects are not secondary consequences of AVG defects and that AVK navigation is independent of AVG. This is consistent with earlier studies, where defects in AVK axon navigation in the left axon tract were also independent of pioneer defects (Steimel et al. 2010; Unsoeld et al. 2012). The idea that aex-3 defects are not limited to the VNC pioneer is strengthened by the observation of synergistic effects in aex-3; nid-1 double mutants in commissural navigation, which is completely independent of AVG navigation. On the other hand, visualization of the entire nervous system suggests that the overall structure of the nervous system is intact in aex-3; nid-1 mutant animals and that the defects are largely limited to the VNC and commissures.
We did not observe any misplacement of neuronal cell bodies (with one exception, see below), indicating that neuronal cell migration is not affected in aex-3; nid-1 double mutants. However, in a small fraction (16%) of animals HSN neurons fail to reach their normal position at the vulva. HSN migration is controlled by Wnt signaling and both egl-20/Wnt and mig-1/Frizzled mutants display similar but more penetrant HSN defects (Pan et al. 2006). Mutations in other Wnts or Frizzled receptors do not cause these defects, but they enhance HSN migration defects of egl-20 and mig-1, suggesting a partially redundant function for Wnts and their receptors in this process (Pan et al. 2006). aex-3 so far has not been linked to Wnt signaling. However, LIN-44/Wnt has been proposed to control sorting of presynaptic RAB-3 to axons and precludes its entry into the dendrite (Poon et al. 2008). This raises the possibility that HSN migration defects in aex-3(hd148); nid-1 mutant animals could be a secondary consequence of the observed mislocalization of RAB-3.
AEX-3 and its interacting partners in AVG axon navigation
AEX-3 activates RAB-3 and AEX-6/Rab27 to regulate synaptic transmission (Mahoney et al. 2006) and physically interacts with CAB-1 to control the defacation motor program (Iwasaki and Toyonaga 2000). Moreover, aex-3 genetically interacts with unc-31 and unc-64 in the context of dauer formation (Iwasaki et al. 1997). Taken together this indicates that AEX-3 regulates multiple processes through different downstream effectors. We found that rab-3 but not aex-6/Rab27 mutant animals have AVG axon cross-over defects in a nid-1 mutant background. Our genetic interaction data suggest aex-3 and rab-3 act in the same genetic pathway to affect AVG navigation and that aex-6/Rab27 is not required for this process. Activated RAB-3 binds to synaptic precursor vesicles (Mahoney et al. 2006), which are transported from the cell body to synapses by the Kinesin-3 motor UNC-104 (Hall and Hedgecock 1991), which is also involved in the anterograde transport of dense core vesicles (DCVs) (Zahn et al. 2004). We observed some AVG axon cross-over defects in unc-104; nid-1 mutant animals, but significantly fewer compared to either aex-3; nid-1 or rab-3; nid-1 mutants, which are not further enhanced in aex-3; unc-104; nid-1 triple mutant animals. This suggests that while aex-3 might be involved in UNC-104-mediated vesicle transport in the context of axonal navigation, these vesicles are likely not exclusively transported by UNC-104. The C. elegans genome encodes 21 kinesins involved in transport, spindle movement, and chromosome segregation (Siddiqui 2002). UNC-116/KIF5 is the kinesin heavy chain anterograde motor protein involved in the transport of synaptic vesicle components and glutamate receptors (Patel et al. 1993; Sakamoto et al. 2005; Hoerndli et al. 2013). VAB-8, an atypical kinase, which controls the posteriorly directed cell migrations and axon outgrowth, also regulates the levels of axon guidance receptors UNC-40/DCC and SAX-3/Robo in neurons (Wightman et al. 1996; Levy-Strumpf and Culotti 2007; Watari-Goshima et al. 2007). Moreover, the actin based minus-end-directed motor Myosin V1 transports both dendritic and axonal surface proteins (Lewis et al. 2011). It is likely that some of these other motor proteins contribute to the vesicular transport required for proper AVG axon navigation. Synaptic vesicles are made competent for fusion by the Rab3 effector molecule UNC-10/Rim (Koushika et al. 2001). We did not observe AVG axon cross-over defects in the unc-10; nid-1 mutant animals, suggesting that unc-10/Rim and by inference synaptic vesicle release is not involved in AVG axon navigation. Since the AVG axon extends before synapses are formed, this is not unexpected and indicates that the role of aex-3 and rab-3 here is independent of synaptic vesicle release.
Both unc-31 and ida-1, which are required for dense core vesicle release (Cai et al. 2004; Speese et al. 2007), have AVG axon cross-over defects in a nid-1 mutant background. Both genes as well as UNC-64/Syntaxin, a component of the SNARE complex (Saifee et al. 1998) required for synaptic and DCV release (Singer-Lahat et al. 2008), act in the same pathway as aex-3. Since neither mature synaptic vesicles nor mature DCVs are expected to be found in neurons at the beginning of axonal outgrowth, it seems more likely that these proteins are involved in the release of some precursor vesicles in the growth cone during AVG axon outgrowth. Since both unc-31; nid-1 and unc-64; nid-1 double mutants have less penetrant AVG defects than aex-3; nid-1 double mutants, it is possible two different populations of vesicles are involved. Alternatively unc-31 and unc-64 might have a partially redundant role in the release of a single type of precursor vesicle. It has been shown that mature synaptic vesicles from vertebrate cultured neurons are different both in function and composition than the vesicles found in growth cones. The SNARE complex proteins are present in both, but are regulated differently (Igarashi et al. 1997). The SNARE complex proteins and Rab3a appear early in the growth cone (Igarashi et al. 1997).
There are at least two possible functions for vesicles in the growth cone in the context of axon navigation. First they can deliver molecules essential for navigation, such as receptors for guidance molecules, to the membrane of the growth cone. We found that UNC-5, one of the receptors for the guidance cue UNC-6/Netrin, acts in the same pathway as AEX-3 in AVG axon navigation. This raises the possibility that AEX-3 is involved in the transport of vesicles carrying UNC-5 to the growth cone. An UNC-5::GFP reporter appears in punctate form in growth cones and axons, suggesting localization to vesicles (Ogura and Goshima 2006; Norris et al. 2014). UNC-51, a serine/threonine kinase and UNC-14, a RUN (RPIP8, UNC-14, and NESCA) domain-containing protein have been proposed to cooperate with an unknown motor protein to regulate the formation, processing, and transport of UNC-5-containing vesicles (Ogura and Goshima 2006).
A second possible role of vesicles in axon guidance arises from the observation that Syntaxin1 associates with DCC (the other Netrin receptor) at the growth cone. This interaction is required for UNC-6/Netrin-dependent migration of axons (Cotrufo et al. 2012). Localized insertion of membrane at sites of activation of guidance receptors is thought to be the key function of this interaction (Tojima et al. 2011; Tojima and Kamiguchi 2015). This raises the possibility that aex-3 is involved in the localized insertion of membrane into the growth cone in response to activation of UNC-5. Distinguishing between these two models would require in vivo observations of growth cones, which unfortunately is not possible for AVG.
In summary, we found that AEX-3 is required for axon guidance in pioneer neurons during nervous system development in C. elegans. aex-3 genetically interacts with rab-3, several genes controlling vesicle release in neurons, and the axon guidance receptor unc-5 (Figure 9). aex-3 is likely involved in transport of vesicles to the growth cone and/or release of vesicles at the growth cone. It could control delivery and/or insertion of UNC-5 protein into the membrane of the growth cone or be involved in targeted insertion of membrane after receptor activation. Although the conventional role for UNC-5 is to mediate repulsion away from the ventral UNC-6/Netrin source, recent studies have also demonstrated a redundant role in attracting some growth cones toward the ventral side (Kulkarni et al. 2013; Levy-Strumpf and Culotti 2014). Moreover, UNC-5 acts redundantly with Wnt signaling to regulate anterior–posterior guidance of neurons and distal tip cells of gonad (Levy-Strumpf and Culotti 2014). It is therefore conceivable that unc-5 plays a partially redundant role in posterior navigation of the AVG axon. Given the evolutionary conservation of all genes we found to be involved in this process, it seems likely that AEX-3 and RAB-3 homologs have a similar role in mammalian nervous systems.
Figure 9.
The aex-3 pathway in AVG axon navigation. The figure describes the observed genetic interactions in the context of the known molecular functions of the proteins involved (e.g., aex-3 is upstream of rab-3 because it is known to activate rab-3).
Acknowledgments
We thank members of the H.H. laboratory and Dr. M. Silverman for comments on the manuscript. The aex-3 cDNA was kindly provided by Dr. E. Jorgensen, the lin-11 promoter construct by Dr. B. Gupta, and the UNC-5::GFP strain by Dr. J.G. Culotti. The aex-3 mutant strain was sequenced at Dr. D.G. Moerman’s laboratory at the University of British Columbia. Some of the nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources. This work was supported by Canadian Institutes of Health Research operating grant 93719 to H.H. Strains are available upon request.
Footnotes
Communicating editor: M. V. Sundaram
Literature Cited
- Ackley B. D., Kang S. H., Crew J. R., Suh C., Jin Y., et al. , 2003. The basement membrane components nidogen and type XVIII collagen regulate organization of neuromuscular junctions in Caenorhabditis elegans. J. Neurosci. 23: 3577–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat J. M., Pan J., Hutter H., 2015. PLR-1, a putative E3 ubiquitin ligase, controls cell polarity and axonal extensions in C. elegans. Dev. Biol. 398: 44–56. [DOI] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T., Fukushige T., Notkins A. L., Krause M., 2004. Insulinoma-associated protein IA-2, a vesicle transmembrane protein, genetically interacts with UNC-31/CAPS and affects neurosecretion in Caenorhabditis elegans. J. Neurosci. 24: 3115–3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. S., Zheng H., Su M. W., Wilk R., Killeen M. T., et al. , 1996. UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell 87: 187–195. [DOI] [PubMed] [Google Scholar]
- Chitnis A. B., Kuwada J. Y., 1991. Elimination of a brain tract increases errors in pathfinding by follower growth cones in the zebrafish embryo. Neuron 7: 277–285. [DOI] [PubMed] [Google Scholar]
- Colavita A., Krishna S., Zheng H., Padgett R. W., Culotti J. G., 1998. Pioneer axon guidance by UNC-129, a C. elegans TGF-beta. Science 281: 706–709. [DOI] [PubMed] [Google Scholar]
- Cornel E., Holt C., 1992. Precocious pathfinding: retinal axons can navigate in an axonless brain. Neuron 9: 1001–1011. [DOI] [PubMed] [Google Scholar]
- Cotrufo T., Andres R. M., Ros O., Perez-Branguli F., Muhaisen A., et al. , 2012. Syntaxin 1 is required for DCC/Netrin-1-dependent chemoattraction of migrating neurons from the lower rhombic lip. Eur. J. Neurosci. 36: 3152–3164. [DOI] [PubMed] [Google Scholar]
- Durbin, R. M., 1987 Studies on the development and organisation of the nervous system of Caenorhabditis elegans. Ph.D. Thesis, University of Cambridge, Cambridge, UK. [Google Scholar]
- Eisen J. S., Pike S. H., Debu B., 1989. The growth cones of identified motoneurons in embryonic zebrafish select appropriate pathways in the absence of specific cellular interactions. Neuron 2: 1097–1104. [DOI] [PubMed] [Google Scholar]
- Garriga G., Desai C., Horvitz H. R., 1993. Cell interactions control the direction of outgrowth, branching and fasciculation of the HSN axons of Caenorhabditis elegans. Development 117: 1071–1087. [DOI] [PubMed] [Google Scholar]
- Hall D. H., Hedgecock E. M., 1991. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell 65: 837–847. [DOI] [PubMed] [Google Scholar]
- Hedgecock E. M., Culotti J. G., Hall D. H., 1990. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 4: 61–85. [DOI] [PubMed] [Google Scholar]
- Hidalgo A., Brand A. H., 1997. Targeted neuronal ablation: the role of pioneer neurons in guidance and fasciculation in the CNS of Drosophila. Development 124: 3253–3262. [DOI] [PubMed] [Google Scholar]
- Hoerndli F. J., Maxfield D. A., Brockie P. J., Mellem J. E., Jensen E., et al. , 2013. Kinesin-1 regulates synaptic strength by mediating the delivery, removal, and redistribution of AMPA receptors. Neuron 80: 1421–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutagalung A. H., Novick P. J., 2011. Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 91: 119–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter H., 2003. Extracellular cues and pioneers act together to guide axons in the ventral cord of C. elegans. Development 130: 5307–5318. [DOI] [PubMed] [Google Scholar]
- Igarashi M., Tagaya M., Komiya Y., 1997. The soluble N-ethylmaleimide-sensitive factor attached protein receptor complex in growth cones: molecular aspects of the axon terminal development. J. Neurosci. 17: 1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii N., Wadsworth W. G., Stern B. D., Culotti J. G., Hedgecock E. M., 1992. UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron 9: 873–881. [DOI] [PubMed] [Google Scholar]
- Iwasaki K., Toyonaga R., 2000. The Rab3 GDP/GTP exchange factor homolog AEX-3 has a dual function in synaptic transmission. EMBO J. 19: 4806–4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki K., Staunton J., Saifee O., Nonet M., Thomas J. H., 1997. aex-3 encodes a novel regulator of presynaptic activity in C. elegans. Neuron 18: 613–622. [DOI] [PubMed] [Google Scholar]
- Kang S. H., Kramer J. M., 2000. Nidogen is nonessential and not required for normal type IV collagen localization in Caenorhabditis elegans. Mol. Biol. Cell 11: 3911–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshishian H., Bentley D., 1983. Embryogenesis of peripheral nerve pathways in grasshopper legs. III. Development without pioneer neurons. Dev. Biol. 96: 116–124. [DOI] [PubMed] [Google Scholar]
- Kim S., Wadsworth W. G., 2000. Positioning of longitudinal nerves in C. elegans by nidogen. Science 288: 150–154. [DOI] [PubMed] [Google Scholar]
- Klose M., Bentley D., 1989. Transient pioneer neurons are essential for formation of an embryonic peripheral nerve. Science 245: 982–984. [DOI] [PubMed] [Google Scholar]
- Koushika S. P., Richmond J. E., Hadwiger G., Weimer R. M., Jorgensen E. M., et al. , 2001. A post-docking role for active zone protein Rim. Nat. Neurosci. 4: 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni G., Xu Z., Mohamed A. M., Li H., Tang X., et al. , 2013. Experimental evidence for UNC-6 (netrin) axon guidance by stochastic fluctuations of intracellular UNC-40 (DCC) outgrowth activity. Biol. Open 2: 1300–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung-Hagesteijn C., Spence A. M., Stern B. D., Zhou Y., Su M. W., et al. , 1992. UNC-5, a transmembrane protein with immunoglobulin and thrombospondin type 1 domains, guides cell and pioneer axon migrations in C. elegans. Cell 71: 289–299. [DOI] [PubMed] [Google Scholar]
- Levy-Strumpf N., Culotti J. G., 2007. VAB-8, UNC-73 and MIG-2 regulate axon polarity and cell migration functions of UNC-40 in C. elegans. Nat. Neurosci. 10: 161–168. [DOI] [PubMed] [Google Scholar]
- Levy-Strumpf N., Culotti J. G., 2014. Netrins and Wnts function redundantly to regulate antero-posterior and dorso-ventral guidance in C. elegans. PLoS Genet. 10: e1004381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T. L., Jr, Mao T., Arnold D. B., 2011. A role for myosin VI in the localization of axonal proteins. PLoS Biol. 9: e1001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone, D., 1991 Studies on the unc-31 Gene of Caenorhabditis elegans. Ph.D. Thesis, University of Cambridge, Cambridge, UK. [Google Scholar]
- MacNeil L. T., Hardy W. R., Pawson T., Wrana J. L., Culotti J. G., 2009. UNC-129 regulates the balance between UNC-40 dependent and independent UNC-5 signaling pathways. Nat. Neurosci. 12: 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney T. R., Liu Q., Itoh T., Luo S., Hadwiger G., et al. , 2006. Regulation of synaptic transmission by RAB-3 and RAB-27 in Caenorhabditis elegans. Mol. Biol. Cell 17: 2617–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell S. K., Ghosh A., Shatz C. J., 1989. Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science 245: 978–982. [DOI] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V., 1991. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat L. L., Robinson R. E., Bakoulis A., Clark S. G., 2014. The conserved transmembrane RING finger protein PLR-1 downregulates Wnt signaling by reducing Frizzled, Ror and Ryk cell-surface levels in C. elegans. Development 141: 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M. L., Staunton J. E., Kilgard M. P., Fergestad T., Hartwieg E., et al. , 1997. Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. J. Neurosci. 17: 8061–8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris A. D., Sundararajan L., Morgan D. E., Roberts Z. J., Lundquist E. A., 2014. The UNC-6/Netrin receptors UNC-40/DCC and UNC-5 inhibit growth cone filopodial protrusion via UNC-73/Trio, Rac-like GTPases and UNC-33/CRMP. Development 141: 4395–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura K., Goshima Y., 2006. The autophagy-related kinase UNC-51 and its binding partner UNC-14 regulate the subcellular localization of the Netrin receptor UNC-5 in Caenorhabditis elegans. Development 133: 3441–3450. [DOI] [PubMed] [Google Scholar]
- Pan C. L., Howell J. E., Clark S. G., Hilliard M., Cordes S., et al. , 2006. Multiple Wnts and frizzled receptors regulate anteriorly directed cell and growth cone migrations in Caenorhabditis elegans. Dev. Cell 10: 367–377. [DOI] [PubMed] [Google Scholar]
- Patel N., Thierry-Mieg D., Mancillas J. R., 1993. Cloning by insertional mutagenesis of a cDNA encoding Caenorhabditis elegans kinesin heavy chain. Proc. Natl. Acad. Sci. USA 90: 9181–9185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon V. Y., Klassen M. P., Shen K., 2008. UNC-6/netrin and its receptor UNC-5 locally exclude presynaptic components from dendrites. Nature 455: 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saifee O., Wei L., Nonet M. L., 1998. The Caenorhabditis elegans unc-64 locus encodes a syntaxin that interacts genetically with synaptobrevin. Mol. Biol. Cell 9: 1235–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto R., Byrd D. T., Brown H. M., Hisamoto N., Matsumoto K., et al. , 2005. The Caenorhabditis elegans UNC-14 RUN domain protein binds to the kinesin-1 and UNC-16 complex and regulates synaptic vesicle localization. Mol. Biol. Cell 16: 483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui S. S., 2002. Metazoan motor models: kinesin superfamily in C. elegans. Traffic 3: 20–28. [DOI] [PubMed] [Google Scholar]
- Singer-Lahat D., Chikvashvili D., Lotan I., 2008. Direct interaction of endogenous Kv channels with syntaxin enhances exocytosis by neuroendocrine cells. PLoS One 3: e1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speese S., Petrie M., Schuske K., Ailion M., Ann K., et al. , 2007. UNC-31 (CAPS) is required for dense-core vesicle but not synaptic vesicle exocytosis in Caenorhabditis elegans. J. Neurosci. 27: 6150–6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimel A., Wong L., Najarro E. H., Ackley B. D., Garriga G., et al. , 2010. The Flamingo ortholog FMI-1 controls pioneer-dependent navigation of follower axons in C. elegans. Development 137: 3663–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. H., 1990. Genetic analysis of defecation in Caenorhabditis elegans. Genetics 124: 855–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojima T., Kamiguchi H., 2015. Exocytic and endocytic membrane trafficking in axon development. Dev. Growth Differ. 57: 291–304. [DOI] [PubMed] [Google Scholar]
- Tojima T., Hines J. H., Henley J. R., Kamiguchi H., 2011. Second messengers and membrane trafficking direct and organize growth cone steering. Nat. Rev. Neurosci. 12: 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsoeld T., Park J. O., Hutter H., 2012. Discoidin domain receptors guide axons along longitudinal tracts in C. elegans. Dev. Biol. 374: 142–152. [DOI] [PubMed] [Google Scholar]
- Wada M., Nakanishi H., Satoh A., Hirano H., Obaishi H., et al. , 1997. Isolation and characterization of a GDP/GTP exchange protein specific for the Rab3 subfamily small G proteins. J. Biol. Chem. 272: 3875–3878. [DOI] [PubMed] [Google Scholar]
- Wadsworth W. G., 2002. Moving around in a worm: netrin UNC-6 and circumferential axon guidance in C. elegans. Trends Neurosci. 25: 423–429. [DOI] [PubMed] [Google Scholar]
- Wadsworth W. G., Bhatt H., Hedgecock E. M., 1996. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron 16: 35–46. [DOI] [PubMed] [Google Scholar]
- Walent J. H., Porter B. W., Martin T. F., 1992. A novel 145 kd brain cytosolic protein reconstitutes Ca(2+)-regulated secretion in permeable neuroendocrine cells. Cell 70: 765–775. [DOI] [PubMed] [Google Scholar]
- Watari-Goshima N., Ogura K., Wolf F. W., Goshima Y., Garriga G., 2007. C. elegans VAB-8 and UNC-73 regulate the SAX-3 receptor to direct cell and growth-cone migrations. Nat. Neurosci. 10: 169–176. [DOI] [PubMed] [Google Scholar]
- White J., Southgate E., Thomson J., Brenner S., 1986. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond., B 314: 1–340. [DOI] [PubMed] [Google Scholar]
- White J. G., Southgate E., Thomson J. N., Brenner S., 1976. The structure of the ventral nerve cord of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 275: 327–348. [DOI] [PubMed] [Google Scholar]
- Wightman B., Clark S. G., Taskar A. M., Forrester W. C., Maricq A. V., et al. , 1996. The C. elegans gene vab-8 guides posteriorly directed axon outgrowth and cell migration. Development 122: 671–682. [DOI] [PubMed] [Google Scholar]
- Zahn T. R., Angleson J. K., MacMorris M. A., Domke E., Hutton J. F., et al. , 2004. Dense core vesicle dynamics in Caenorhabditis elegans neurons and the role of kinesin UNC-104. Traffic 5: 544–559. [DOI] [PubMed] [Google Scholar]
- Zallen J. A., Yi B. A., Bargmann C. I., 1998. The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell 92: 217–227. [DOI] [PubMed] [Google Scholar]
- Zallen J. A., Peckol E. L., Tobin D. M., Bargmann C. I., 2000. Neuronal cell shape and neurite initiation are regulated by the Ndr kinase SAX-1, a member of the Orb6/COT-1/warts serine/threonine kinase family. Mol. Biol. Cell 11: 3177–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M., McBride H., 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2: 107–117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.