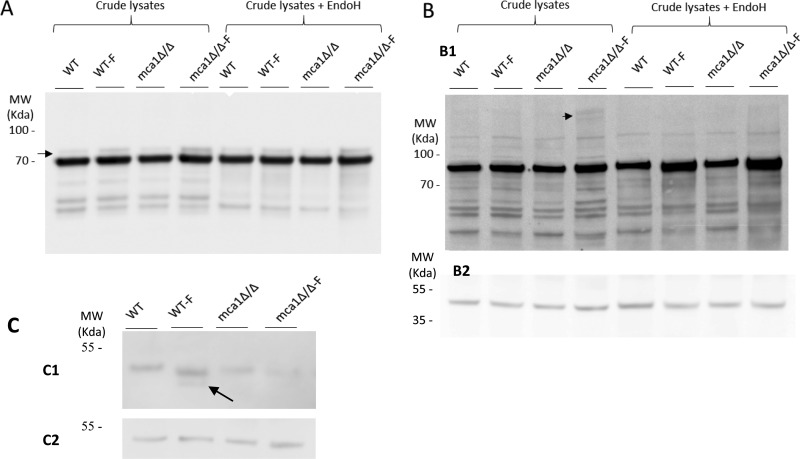
Fig. 6.
Validation of the induction of Ssb1p and Cdc48p glycosylation in the mca1Δ/Δ-F extract and immunocharacterization of a putative substrate of Mca1p. The abundances of Ssb1p (A) and Cdc48p (B) were assessed in immunoassays, as a function of cell conditions (WT, WT-F, mca1Δ/Δ and mca1Δ/Δ-F) in samples treated or not by Endo H. Arrows indicate immunoreactive material of the higher mass, attributed to some glycosylated forms of the proteins. Those were Endo H insensitive (Ssb1p, panel A) or Endo H sensitive (Cdc48p, panel B). Protein loading are probed using anti-Pgk1 antibodies (B2). C, Immunodetection of Bgl2p in the different experimental conditions. The arrow points to a degradation product observed only in the WT-F condition. Control of protein loading using the anti-Pgk1p antibody is presented in C2.