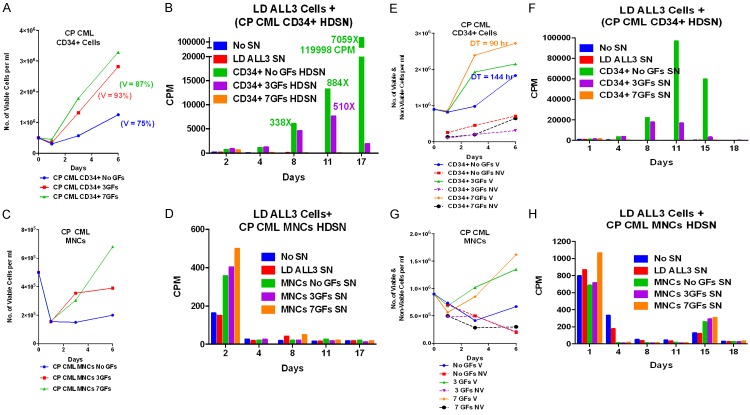
Figure 11.
Stimulatory effects of CP CML MNC and enriched CD34+ cells on the growth of ALL3 cells at LD using [3H]-thymidine uptake assay. (A and C) Comparison of the growth of CP CML MNCs and enriched CD34+ cells which were obtained from fresh/frozen leukapheresis sample from a newly diagnosed patient with CP CML presenting with a WBC of 700,000/mm3. Aliquots of the frozen MNC were thawed, highly enriched for CD34+ cells, and the growth of the MNCs and CD34+ cells at a HD (5 × 105/ml) in QBSF-60 with and without GFs stimulation was compared. The CD34+ cells grew about twice as fast with GFs stimulation as those without GFs while the MNCs hardly grew at all. (B and D) The supernates from these two HD cultures (A and C) were collected after 6 days to determine if they would stimulate the growth of LD ALL3 cells (5000 cells/ml) at which they wouldn’t grow without stimulation as measured by [3H]-thymidine uptake assay. The representative numbers indicate the fold increase in uptake on that particular day of the cells growing with added HDSN compared to the control LD ALL3 cells with no HDSN. The very slow growing HD CD34+ without GFs were by far the most stimulatory with the maximum increased fold difference in CPM between the control and stimulated ALL3 cells (2059x) occurring on day 17. The greater stimulatory activity of the HDSN of the CD34+ cells without GFs was probably because the GFs induced the majority of the CP CML cells to begin differentiating and the differentiated cells were no longer producing the same proliferation triggering factors secreted by the more primitive CML CD34+ S/P cells. The MNCs hardly grew and their HDSN didn’t stimulate the LD ALL3 cells except minimally on day 2. (E-H) Repeat experiment similar to (A-D). Because of the LD ALL3 cells stimulated by the HDSN of the CD34+ cells with no GFs were strikingly stimulated, this experiment was repeated using the same protocol and the same CML cells obtained from another frozen-thawed aliquot from the same patient except the CD34+ and MNCs cultures were started at an even higher cell density (9 × 105 cells/ml). The results were similar except that the maximum fold increase in CPM of the CD34+ cells with no GFs occurred sooner on day 11 and was lower (2247x control) than in the first experiment. The same CD34+ cells starting at almost twice the cell density as in the first experiment grew more slowly, and neared saturation density sooner so they probably were producing less stimulatory factors by day 6 when the HDSNs were collected.