Figure 4.
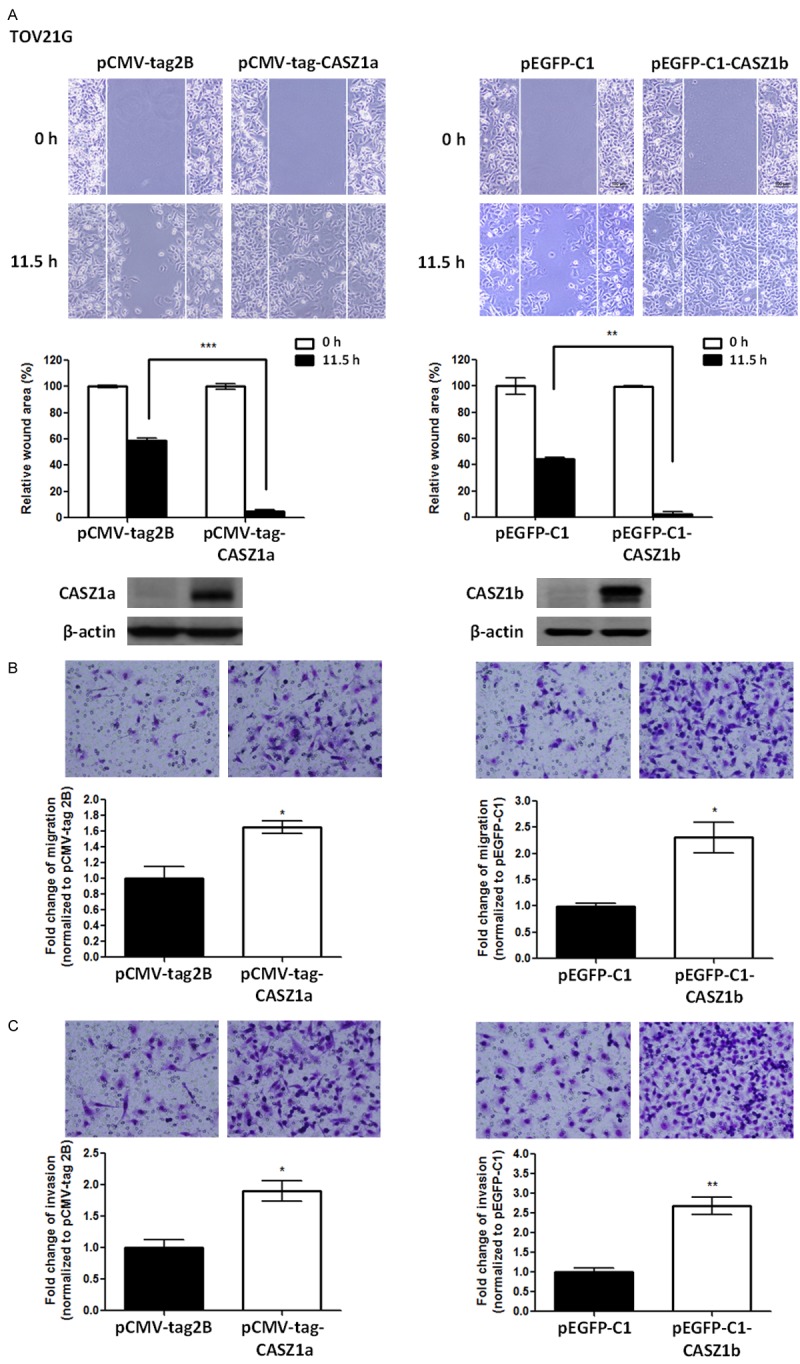
Overexpression of either CASZ1a or CASZ1b enhances migration and invasion in TOV21G cells. A. Both CASZ1a and CASZ1b promote cell migration in TOV21G cells, as demonstrated by the wound healing assay. TOV21G cells were electroporated with the pCMV-tag2B, pCMA-tag-CASZ1a (left panel), pEGFP-C1 vector, or pEGFP-C1-CASZ1b vector (right panel). Twenty-four hours later, 2 × 104 of the indicated cells were seeded on ibidi culture inserts. The wound was imaged (upper panel) at 0 h and 11.5 h after the removal of culture inserts, and the wound area was measured using ImageJ (n ≥ 3 per group). The wound area at 11.5 h was normalized to the wound area at 0 h (middle panel). CASZ1a and CASZ1b expression was analyzed using immunoblotting assays (lower panel). β-actin was used as the internal control. B. Both CASZ1a and CASZ1b promote cell migration in TOV21G cells, as demonstrated using the transwell migration assay. Briefly, 2 × 104 of the indicated cells were seeded on transwells and incubated for 6 h. The cells that had migrated through the transwell were counted (n = 3 per group), and the values were normalized to the vector control group to calculate relative migration ability. C. The effect of CASZ1a or CASZ1b on cell invasion in TOV21G cells was determined using the in vitro transwell invasion assay. Briefly, 1 × 105 cells were seeded on transwells coated with 100 μg of Matrigel and incubated for 24 h. The cells that had invaded the membrane were counted (n = 3 per group), and the values were normalized to the vector control group to calculate relative invasive ability. The error bars represent the SEM. *P < 0.05; **P < 0.005; ***P < 0.001 by Student’s t-test.
