Figure 2. Series 1 compounds show stereoselective inhibition of the RSV replicon.
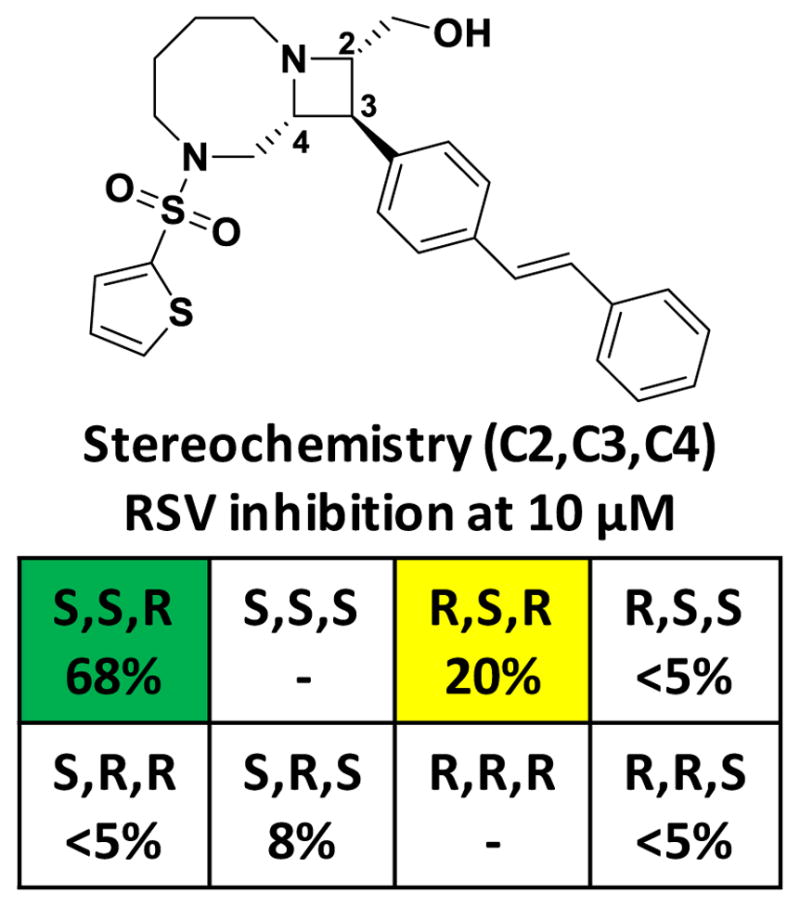
HTS data from the RSV replicon data are shown for all available stereoisomers of a representative compound from Series 1 (BRD9101). The stereochemistry of C2, C3, and C4 is indicated, along with the % inhibition of the RSV replicon; the S,S,R stereoisomer was the most potent, while the R,S,R diastereomer, differeing only in the extracyclic hydroxyl group, showed more modest inhibition. All other tested diastereomers were inactive.
