Abstract
Endometriosis is a chronic disease in which epithelial and stromal cells that resemble the eutopic endometrium are found in ectopic lesions. In order to examine how microenvironmental factors such as extracellular matrix and macrophages influence disease progression, 12Z (an immortalized ectopic epithelial cell line) were cultured on tissue culture plastic (TCP) or in gels of recombinant basement membrane (rBM) or collagen I. Unlike cells in other conditions, cells in rBM formed multi-cellular structures in a 67 kDa non-integrin laminin receptor (67LR)-dependent manner. To examine the impact of macrophage-secreted factors on cell behavior, 12Z cells on all three substrates were treated with conditioned media from differentiated THP-1 (an immortalized monocytic cell line). Significant proliferation and invasion was observed only with cells cultured in rBM, indicating that extracellular matrix cues help dictate cell response to soluble signals. Cells cultured on rBM were then treated with individual cytokines detected in the conditioned media, with increased proliferation observed following exposure to interleukin-8 (CXCL8/IL-8) and both increased proliferation and invasion following treatment with heparin-binding EGF-like growth factor (HB-EGF). This study suggests that rBM gels can be used to induce in vitro lesion formation in order to identify soluble factors that influence proliferation and invasion.
Key Terms: endometriosis, 3D culture, Heparin-binding EGF-like growth factor (HB-EGF), CXCL8, basement membrane
Introduction
Endometriosis is a gynecological condition that affects approximately 10% of women and is associated with pelvic pain and infertility.1 Despite its prevalence and significant impact on women’s health, a lack of appropriate in vitro and in vivo model systems has resulted in limited knowledge about specific mechanisms that can be targeted to slow disease progression or treat the associated infertility. The defining feature of endometriosis is the presence of ectopic lesions composed of epithelial cells, stromal cells, and extracellular matrix (ECM) that resemble the eutopic endometrium. Currently, the standard of care includes surgical removal of lesions, pain medication, hormonal intervention (e.g., oral contraceptives), or, possibly, removal of the uterus and ovaries, resulting in premature menopause.2
The study of endometriosis has been hampered by a lack of relevant model systems and most in vitro studies have been performed using standard tissue culture substrates, despite substantial evidence in other disease models that cell behavior is significantly impacted by exposure to ECM proteins and culture in three-dimensional (3D) environments. Cells interact with ECM proteins primarily through integrin receptors, which activate downstream signaling cascades to direct gene expression and cell behaviors such as proliferation, apoptosis, migration, and differentiation.3 The specificity of the integrin-ECM interaction can result in different cell responses. For example, immortalized mammary epithelial cells are capable of initiating branching in collagen I, but require basement membrane to undergo full polarization and differentiation.4 In addition, cells cultured in 3D gels of ECM proteins often behave differently than cells cultured on two-dimensional substrates as the 3D matrix provides additional cues that impact cellular organization and response to stimuli.5
The ECM in endometriotic lesions is similar to the eutopic endometrium, with a basement membrane of collagen IV and laminin, and stromal expression of collagen I, fibronectin, and vitronectin.6–8 Despite this similar ECM profile, stromal cells isolated from ectopic lesions have altered integrin expression levels and increased adhesion to adsorbed ECM proteins compared to cells from healthy women.9 ECM has been incorporated in several in vitro models of the eutopic endometrium to examine the interactions between epithelial and stromal cells,10, 11 but the impact of ECM on endometriotic cells and their response to factors produced by macrophages has not been described. In this study, we cultured an immortalized ectopic epithelial cell line (12Z)12 in 3D ECM gels in order to examine the impact of ECM on the endometriotic cells. In vivo, the ECM is a complex mixture of components whose precise composition varies between tissues and likely between patients. Therefore, as a first approximation, we utilized commercially-available recombinant basement membrane (rBM) and collagen I to represent a basement membrane and stromal context, respectively.
Macrophages have been suggested to play an important role in the establishment, maintenance, and growth of endometriotic lesions, based on their elevated levels and the increased concentration of various cytokines in peritoneal fluid of women with the disease.13–17
Depletion of macrophages in a mouse model of endometriosis inhibited the establishment of new lesions and growth of established lesions, providing direct experimental evidence for a role for macrophages.18 In vitro studies also support that macrophages influence cell behavior. Eutopic endometrial epithelial cells proliferated more when co-cultured with macrophages from women with endometriosis compared to healthy controls19 and eutopic endometrial stromal cells treated with conditioned media from macrophages have altered gene expression.20 In these studies, the use of co-culture or conditioned media mimics the in vivo environment where macrophages secrete a number of cytokines simultaneously. While prior studies have not examined the impact of the full set of macrophage-derived factors on ectopic cells, these cells do respond to treatment with individual factors that macrophages produce. For example, ectopic epithelial cells cultured on tissue culture plastic and treated with saturating doses of TNFα had increased secretion of pro- and anti-inflammatory cytokines.21 Using our in vitro models of the basement membrane and stromal context, we examined ectopic epithelial cell response to macrophage-derived factors and determined that these cells were more sensitive in the basement membrane context, showing increased proliferation and invasion compared to cells in a stromal context or on tissue culture plastic.
Materials and Methods
Materials and Cell Culture
12Z ectopic epithelial cells12 were a generous gift of Dr. Anna Starzinski-Powitz (Goethe-Universität Frankfurt) and were isolated under approval by the local ethical committee. 12Z were maintained in culture in DMEM-F12 media supplemented with 10% FBS (Life Technologies, Grand Island, NY). THP-1 cells were obtained from ATCC (Manassas, VA) and maintained in suspension culture in RPMI-1640 supplemented with 10% FBS and 0.05 mM β-mercaptoethanol. Cells were routinely checked and confirmed to be mycoplasma-negative using the MycoAlert kit (Lonza, Basel, Switzerland). Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Analysis of 12Z Adhesion to ECM
To examine the adhesion of 12Z cells, ECM proteins were immobilized on a black-walled 96-well plate as previously described.22 Briefly, ECM proteins (collagen I (6 μg/mL, PureColTM, Advanced Biomatrix, San Diego, CA), collagen IV (6 μg/mL), laminin (6 μg/mL, LN-521TM, BioLamina, Sundbyberg, Sweden), fibrinogen (1 mg/mL), or 1% BSA as a negative control) were dissolved in a 15 mM sodium carbonate, 35 mM sodium bicarbonate buffer (pH 9.6). 100 μL of collagen I, collagen IV, laminin, or BSA were added per well, incubated for 24 hours at 37°C and then washed one time with PBS. 100 μL of fibrinogen was added per well, incubated for 23 hours at 37°C, washed with PBS (3 times for 3 minutes), incubated with 215 U/mL thrombin in 0.1% BSA-PBS for 60 minutes to generate fibrin, and washed with PBS (3 times for 3 minutes). 12Z were seeded in serum-free media (10,000 cells/well) on these surfaces and incubated for 2 hours at 37°C. As a positive control, 12Z cells were also seeded in complete media. Non-adherent cells were removed with a PBS wash and adherent cells were then fixed for 20 min with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 (4 times for 5 min each). Adherent cells were stained with 0.4 μM TO-PRO-3 for 1 hour (Invitrogen), washed with 0.1% Tween in PBS (3 times for 5 min, shaking at 300 rpm), and allowed to dry completely. Plates were scanned using a LI-COR Odyssey Infrared Imaging System and fluorescence was quantified using the Odyssey software (LICOR, Lincoln, NE).
Culture in ECM Gels
12Z cells were cultured in recombinant basement membrane (rBM, growth-factor reduced MatrigelTM, BD Biosciences, San Jose, CA) or collagen type I gels (Coll I, PureColTM) following a previously-described protocol.23 Briefly, 120 μL of soluble rBM was pipetted into a 24-well tissue culture plate well (25–35 μL for 96-well plates) and allowed to gel at 37°C for 30 minutes. Coll I was combined 4:1 with phenol red containing media and neutralized with HEPES and NaOH (to a final concentration of 2.48 mg/mL), then 120 μL was pipetted into a 24-well tissue culture plate well (25–35 μL for 96-well plates) and allowed to gel at 37°C for 60 minutes. 12Z cells were trypsinized, counted by hemocytometer, and resuspended at appropriate concentrations in 250 μL of media (40 μL for 96-well plates) prior to gently pipetting onto the gelled ECM. For initial experiments, cell density was varied from 5,000–175,000 cells/cm2. Following a 30 minute incubation at 37°C, an overlay of 5% ECM in media was added by pipetting an equal volume of 10% ECM diluted in media to the side of each well. rBM is recognized to be a mixture of components (61% laminin, 30% collagen IV, and 7% entactin), and as a natural biomaterial shows variability from batch to batch. Therefore, experiments were repeated across different lot numbers of rBM, and showed similar trends.
Fluorescent Staining and Microscopy
Cell morphology was examined by phase microscopy on either a Zeiss Axiovert 40C with AxioCam ICc1 camera, and AxioVision software or an Olympus IX51 with Hamamatsu camera, and SimplePCI6 software. To determine cell viability, cells were stained with 10 μM ethidium homodimer-1 and 0.1 μM calcein AM (Life Technologies) for 40 minutes and imaged on a 3i / Olympus Deconvolution System with Spherical Aberration Correction (fluorescence and phase contrast capabilities), Orca ER camera, and SlideBook imaging software. Time-lapse microscopy was performed for the first 24 hours in culture using an Olympus IX71 inverted microscope with Orca-3 camera, CellSens imaging software, fluorescence, environmentally-controlled incubation chamber, and motorized XYZ stage with auto-Z-focus.
Functional Blocking of ECM Receptors
To determine the effects of blocking cellular interactions with specific rBM components 12Z were trypsinized, resuspended, and incubated with 10 μg/mL anti-α6 integrin function blocking antibody (GoH3, Biolegend, San Diego, CA), 50 μg/mL soluble collagen IV, 30 mM melibiose (D(+)-Melibiose monohydrate, Acros Organics, Fair Lawn, NJ), or a rat IgG2a isotype control (Biolegend) for 45 minutes at room temperature. Cells were then plated in rBM as described above with the exception that each overlay was modified to include the blocking treatments and imaged at 24 hours. To quantify the effect of each inhibitor, images were taken in the middle of each well (n=3) and clusters were binned as either small clusters (2–5 cells/cluster) or large clusters (>6 cells/cluster).
Treatment with THP-1 Conditioned Media and Cytokines
To examine the impact of cytokines and growth factors secreted by macrophages, THP-1 were plated at a density of 1.2 × 106 cells/well in 6-well plates and stimulated with 100 ng/mL of phorbol 12-myristate 13-acetate (PMA) for 48 hours, resulting in their differentiation to a macrophage-like state.24 THP-1 cells were then changed to serum-free RPMI-1640 media for 24 hours to produce conditioned media. For control media, the process was mimicked exactly, except without THP-1 cells, so that the conditioned media and control media had similar baseline composition (including any residual PMA). The composition of THP-1 conditioned and control media was analyzed for the presence of 36 cytokines and chemokines by the Human Cytokine Array Kit, Panel A and for levels of ErbB ligands (transforming growth factor-α (TGF-α), neuregulin-1β (NRG-1β, and heparin-binding EGF-like growth factor (HB-EGF)) by ELISA according to manufacturer’s instructions (R&D Systems, Minneapolis, MN).
12Z cells were plated in 96-well plates on tissue culture plastic (1,800 cells/well) or in rBM (13,000 cells/well) or Coll I gels (5,200 cells/well) for 24 hours as described above, serum-starved for 24 hours, and then treated with freshly collected THP-1 conditioned or control media. These densities were empirically selected to result in cell densities that did not reach full confluency during the timecourse of the experiment for the monolayer conditions (tissue culture plastic, Coll I) and formed consistent aggregates for the rBM condition (which does not proliferate to confluency). Cells were retreated 48 hours later with freshly collected THP-1 conditioned or control media. After 48 and 72 total hours of treatment, cell number was quantified by CellTiter Glo assay (Promega, Madison, WI) using a Veritas plate luminometer (Promega). Readings were corrected to a blank of media incubated on the corresponding substrate; blank readings were <0.1% of sample readings. For experiments with individual cytokines, 12Z cells were plated in 96-well plates in rBM gels for 24 hours as described above, serum-starved for 24 hours, and then treated with 100 ng/mL of recombinant CXCL8, IL-1ra, MIF (Peprotech, Rocky Hill, NJ), 10 ng/ml of recombinant HB-EGF (Peprotech, Rocky Hill, NJ), or vehicle control in DMEM-F12. Cells were retreated 48 hours later. After 72 total hours of treatment cell number was quantified by CellTiter Glo.
Statistics
Data was analyzed by t-test, Dunnett’s test for multiple comparisons, or Pearson’s chi-squared test (JMP Pro software version 10.0.0; SAS Institute, Inc.)
Results
Ectopic Epithelial Cells Adhere to Different ECM Proteins
To determine which ECM should be utilized for our 3D in vitro studies, we first characterized which ECM proteins 12Z adhered to by culturing 12Z cells on adsorbed collagen IV, laminin, collagen I, and fibrin, ECM proteins that are commonly used as 3D matrices.25–27 12Z showed the most adhesion on laminin and collagen IV coated surfaces (Figure 1). This finding, combined with reports of a basement membrane in endometriotic lesions,6, 8 motivated the use of recombinant basement membrane as a 3D matrix in these studies. Cells also adhered to collagen I and fibrin (Figure 1), indicating that 12Z cells express a wide range of integrins. As collagen I is a key component of most stromal matrices and has been observed in ectopic lesions,8 it was selected as an additional 3D matrix in which to examine 12Z cell behavior in vitro.
FIGURE 1.
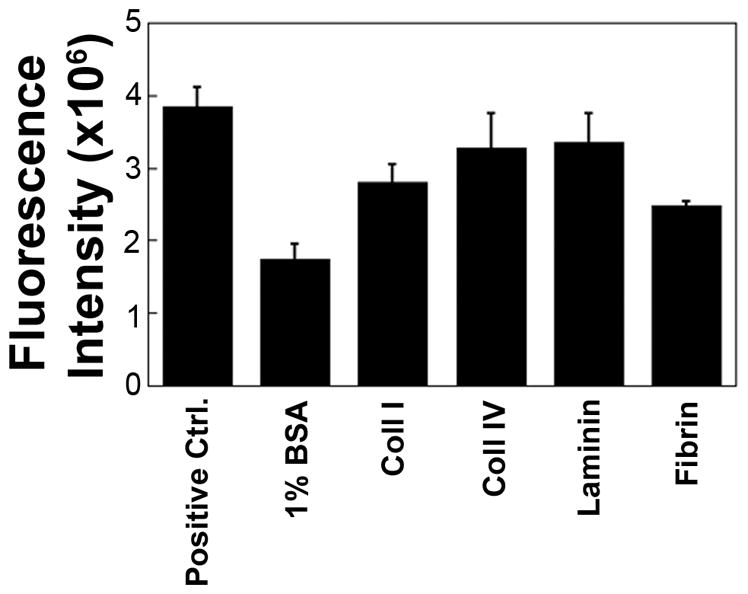
12Z preferentially adhered to laminin and collagen IV, two key components of the basement membrane, with lower levels of adhesion to collagen I and fibrin. 12Z were plated in serum-free media for 2 hours on adsorbed ECM proteins, stained with the nuclear stain TO-PRO-3, and the number of adherent cells was quantified via fluorescence intensity. Cells that were plated in complete media were used as a positive control and wells coated with 1% BSA were used as a negative control.
Ectopic Epithelial Cells Have Distinct Morphologies on Different Substrates
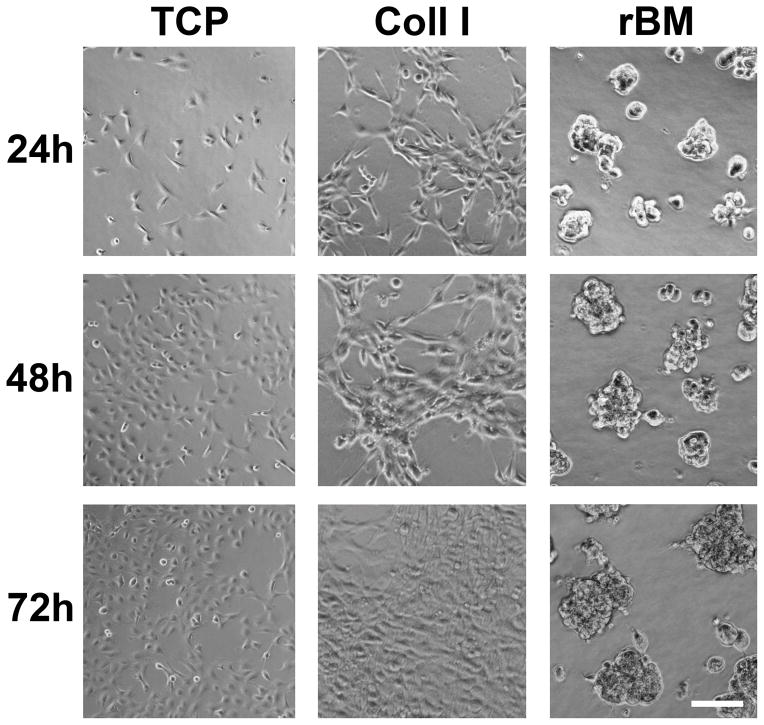
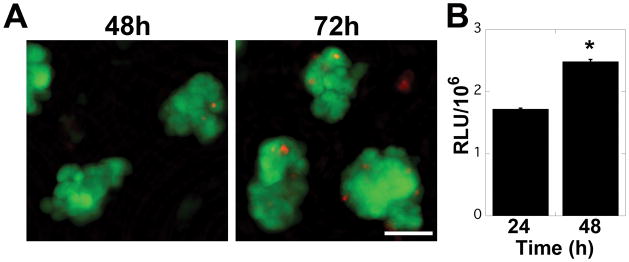
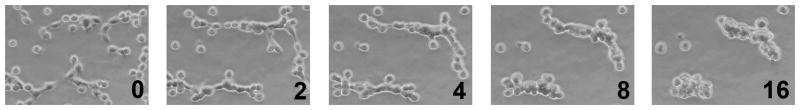
In order to examine the potential impact of 3D ECM on cellular behavior in endometriosis, 12Z cells were cultured on tissue culture plastic (TCP), or in collagen I (Coll I) or recombinant basement membrane (rBM) gels in densities ranging from 5,000 – 175,000 cells/cm2. On TCP, 12Z cells formed a monolayer with a characteristic epithelial morphology at all cell densities tested (Figure 2, 5,000 cells/cm2). On Coll I gels, 12Z initially exhibited a spindle-shaped morphology (in contrast to cells cultured on TCP), but proliferated to form a confluent monolayer with a cobblestone appearance (Figure 2, 25,000 cells/cm2). In contrast, on rBM the cells formed 3D multi-cellular clusters. This behavior was observed for cell densities ranging from 25,000–150,000 cells/cm2, although clusters fused to form an irregular, web-like pattern at higher densities (Figure 2, 50,000 cells/cm2). Clusters were morphologically stable for at least five days in culture, at which time cells in some clusters were observed to invade into the underlying gel (Supplemental Figure 1). Cluster formation appeared to be dependent on the identity of ECM as this behavior was not observed on Coll I gels at any cell density.The multi-cellular clusters that formed in rBM gels were composed primarily of viable cells with occasional dead cells dispersed randomly in the cluster (Figure 3A). In contrast to breast epithelial cells cultured in similar conditions,23 clusters did not appear to have formed a hollow center when examined by multiphoton microscopy (Supplemental Figure 2). Despite the high cellular density that occurred in the three-dimensional clusters, viability remained high throughout the culture period. To further confirm cell viability, proliferation was monitored using CellTiter Glo. Consistent with the increased size of clusters at 48 and 72 hours (Figure 2), 12Z cells proliferated when cultured in rBM gels (Figure 3B). To determine if the multi-cellular structures formed as a result of individual cells rapidly proliferating and forming clusters or from cells migrating and ‘sticking’ together, 12Z cells were plated in rBM gels and imaged using time-lapse microscopy. Analysis of the collected images demonstrated that cells migrated in rBM gels and formed elongated, multi-cellular structures within four hours (Figure 4). During this period of cluster formation, minimal cell proliferation was observed. Over the next twelve hours, these elongated structures compacted to form spherical clusters that were retained over extended time in culture.
FIGURE 2.
12Z ectopic epithelial cells have distinct morphologies when cultured with different substrates. Cells were plated on tissue culture plastic (TCP, 5,000 cells/cm2) or in collagen I (Coll I, 25,000 cells/cm2) or recombinant basement membrane (rBM, 50,000 cells/cm2) gels and observed for 72 hours. Scale bar equals 100 μm.
FIGURE 3.
12Z cells formed viable structures in rBM gels and continued to proliferate. (a) 12Z cells were viable in the multi-cellular structures observed in rBM gels. Cells were plated at a density of 50,000 cells/cm2 and stained with Live/Dead (green/red, respectively) after 48 and 72 hours of culture. Scale bar equals 50 μm. (b) 12Z proliferated in the multi-cellular structures observed in rBM gels. Cells were plated at a density of 50,000 cells/cm2 and cell number was assayed by CellTiter Glo. Data plotted as average + SEM, * indicates significantly different than 24 hours, p<0.05 (n=3).
FIGURE 4.
12Z cells formed multi-cellular structures through migration. Cells were plated in rBM gels at a density of 50,000 cells/cm2 and examined by time-lapse microscopy. Numbers indicate time elapsed in culture in hours (imaging started approximately 30 minutes after the rBM overlay was applied).
Ectopic Epithelial Cells Utilize the 67-kDa Laminin Receptor (67LR) for Cluster Formation
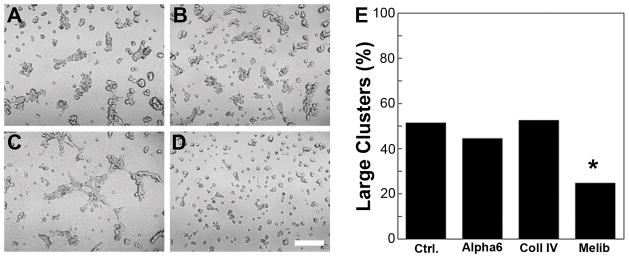
To gain further insight into the mechanism of 12Z cluster formation, cells were treated with inhibitors for receptors known to interact with specific rBM components. These inhibitors included anti-α6 integrin monoclonal antibody (GoH3), full-length soluble collagen IV to block all cell surface receptors to collagen IV,28 and 30 mM melibiose, an antagonist of the 67-kDa laminin receptor (67LR).29 Treatment with the anti-α6 integrin antibody did not result in a noticeable morphological difference compared to the isotype control at 24 hours (Figure 5A, B). Cells treated with exogenous collagen IV formed some clusters but also displayed areas of larger web-like patterns (Figure 5C), suggesting a role for collagen IV in the development of isolated clusters. Finally, treatment with melibiose resulted in significantly fewer large clusters (>5 cells) than the other conditions (Figure 5D, E), suggesting a role for the 67LR in 12Z cluster formation. Treatments with combinations of the inhibitors further support these findings (Supplemental Figure 3).
FIGURE 5.
12Z cells formed significantly fewer large clusters (>5 cells/cluster) when the 67LR was blocked. Cells were pretreated with a) isotype control, b) 10 μg/mL anti-α6 integrin blocking antibody GoH3, c) 50 μg/mL soluble collagen IV, or d) 30 mM melibiose for 45 minutes and then cultured in rBM gels for 24 hours. Scale bar equals 250 μm. e) The effect of each inhibitor was determined by manually binning clusters according to size (2–5 cells = small, >5 cells = large). * indicates significantly different than control as determined by Pearson’s chi-squared analysis, p<0.01.
Ectopic Epithelial Cells in rBM were Sensitive to Macrophage-Derived Soluble Factors
Women with endometriosis have elevated levels of activated macrophages and cytokines, indicating a potential role for macrophage-derived factors in the progression of the disease.13, 14 To examine the interaction between these two cell types in more detail, 12Z cells were cultured on TCP or in Coll I or rBM gels and exposed to conditioned media from THP-1, a monocytic cell line that is frequently used as a model of macrophages. When cultured on rBM, 12Z cells had increased proliferation when treated with THP-1 conditioned media in comparison to control media (Figure 6). This increased proliferation was not observed for cells cultured on TCP or in Coll I, indicating that the ECM environment experienced by ectopic epithelial cells impacts their responsiveness to macrophage-derived stimuli. 12Z cells treated with control media maintained a compact, multi-cellular morphology in rBM gels throughout the culture period (Figure 7), similar to the morphology observed with standard culture media (Figure 2). In contrast, cells treated with THP-1 conditioned media appeared to break out of their clusters, with cells on the edge of clusters extending cellular processes along the surface of or invading further into the gel. After 48 hours of exposure, the cells had clearly invaded into the gel, with cells from the same cluster observed on different focal planes. No obvious morphological changes were observed for 12Z cells cultured on TCP or Coll I, further demonstrating that the response of ectopic epithelial cells was ECM context-dependent (Supplemental Figure 4).
FIGURE 6.
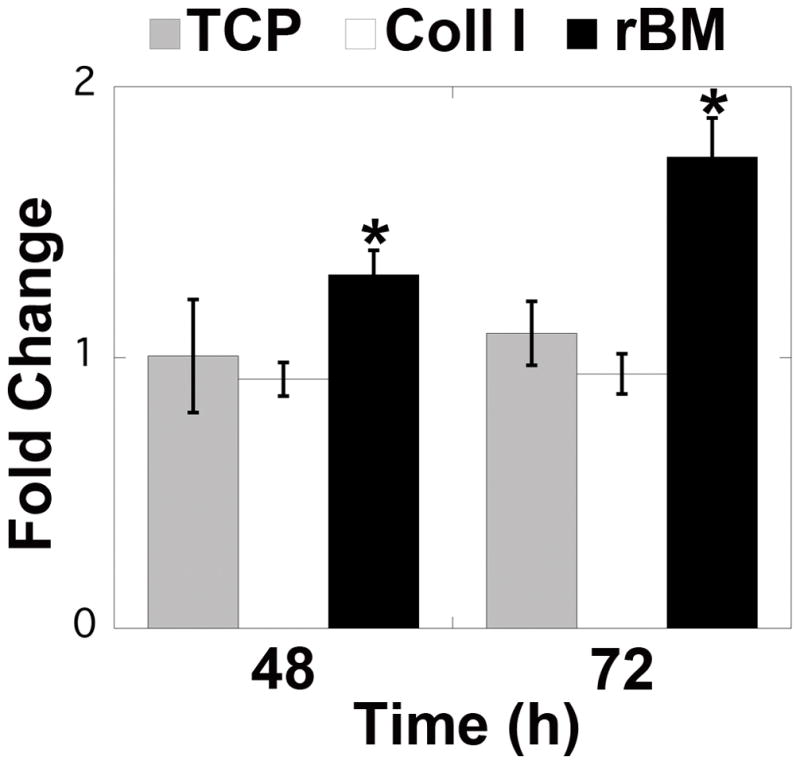
12Z cells proliferated in response to THP-1 conditioned media when cultured in rBM gels. Cells were plated on TCP or in Coll I or rBM gels and treated with THP-1 conditioned media. Cell number was assayed by CellTiter Glo relative to control-treated 12Z cells cultured with the same substrate. Data plotted as average + SEM normalized to control media for the same substrate; * indicates significantly different than control for the same substrate, p<0.05 (n=3–4).
FIGURE 7.
Multi-cellular structures were disrupted after exposure to THP-1 conditioned media. Cells were plated in rBM gels, treated with control or conditioned media, and observed for 72 hours. Scale bar equals 50 μm.
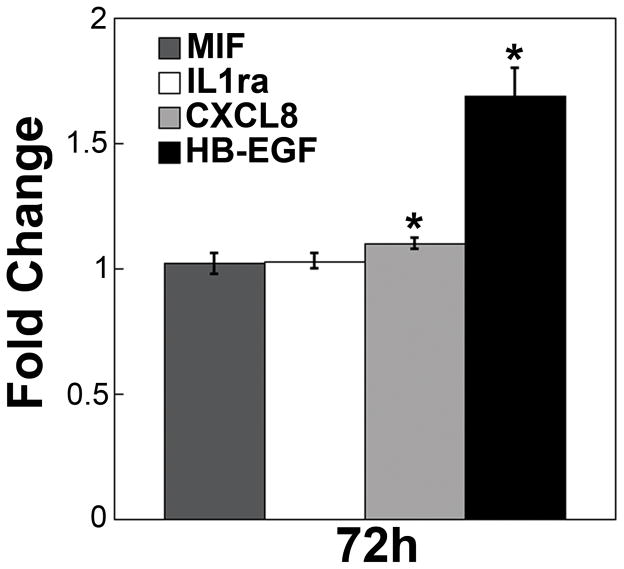
Macrophages have been shown to secrete a number of different growth factors spanning a variety of protein families. To determine which macrophage-secreted factors were responsible for the observed changes in 12Z behavior, THP-1 conditioned media was collected and assayed using an array that screens for 36 different growth factors, cytokines, and chemokines (Supplemental Figure 5). This screen detected three factors – interleukin-8 (CXCL8/IL-8), macrophage migration inhibitory factor (MIF), and interleukin-1 receptor antagonist (IL-1ra). Each of these factors has been reported to be elevated in women with endometriosis and may play a role in disease progression.30–32 Additionally, macrophages have been shown to secrete ErbB ligands, which are known to play a role in cellular proliferation and migration.33 Therefore, we performed ELISAs to determine the levels of three ErbB ligands (TGFα, NRG1β, and HB-EGF) that were not included in the screen. THP-1 conditioned media contained detectable levels of HB-EGF, but not TGFα or NRG1β (Supplemental Table 1). We examined the impact of each individual factor (CXCL8, MIF, IL-1ra, and HB-EGF) on 12Z cells cultured in rBM gels. 12Z cells cultured in rBM proliferated significantly in response to treatment with HB-EGF and CXCL8, with the most substantial impact resulting from HB-EGF (Figure 8). While morphological changes were not observed for treatments with CXCL8, MIF, or IL-1ra (Supplemental Figure 6), cells treated with HB-EGF broke out of their clusters and invaded into the rBM gels (Figure 9).
FIGURE 8.
12Z cells proliferated in response to CXCL8 or HB-EGF when cultured in rBM gels. Cells were plated in rBM gels and treated with 100 ng/mL of CXCL8, MIF, or IL-1ra or 10 ng/mL HB-EGF. Cell number was assayed by CellTiter Glo after 72 hours of treatment relative to vehicle-treated 12Z cells cultured in rBM. Data plotted as average + SEM, * indicates significantly different than vehicle control, p<0.05 (n=4).
FIGURE 9.
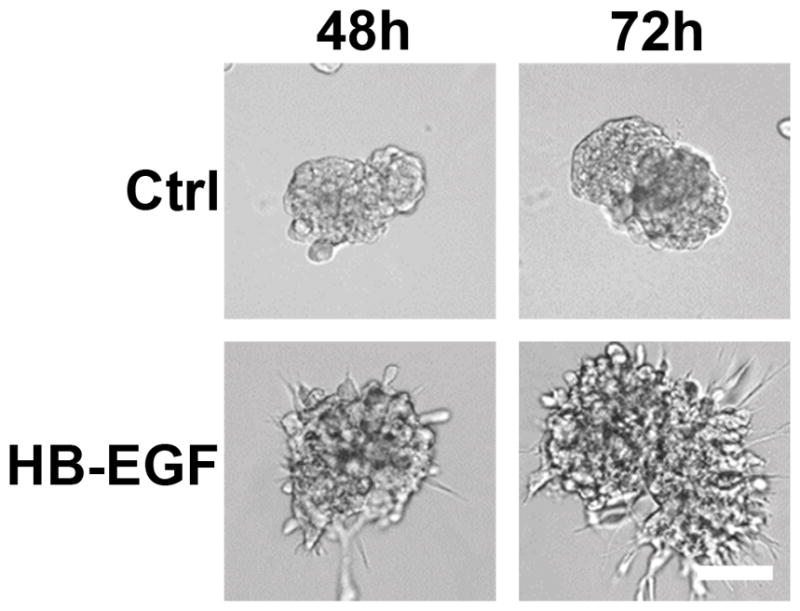
Multi-cellular structures were disrupted after exposure to HB-EGF. Cells were plated in rBM gels, treated with vehicle or 10 ng/mL HB-EGF, and observed for 72 hours. Scale bar equals 50 μm.
Discussion
Analyzing the complex interactions between endometriotic cells and the cellular microenvironment could potentially lead to the identification of new therapeutic targets to slow or stop disease progression. In this study, we utilized an ectopic epithelial cell line (12Z) and analyzed how cellular behavior was influenced by the ECM and factors secreted by macrophages. Cells are capable of adhering to a vast range of ECM proteins depending on their integrin expression. To determine which proteins 12Z interact with we examined 12Z adhesion to adsorbed ECM proteins and found that that the highest number of cells adhered to laminin and collagen IV. As ectopic epithelial cells form glands with a basement membrane in vivo,6 these results suggested that culture in rBM may be a more physiologically relevant model to study endometriosis in vitro. 12Z also adhered well to collagen I, a main component of the stromal microenvironment.34 Therefore, we decided to culture cells in overlay gels of either rBM extract or collagen I to mimic the basement membrane and stromal environments, respectively. Our results demonstrated that the ECM influenced cellular morphology and interactions, as 12Z formed multi-cellular clusters of rounded cells in rBM overlay gels and a monolayer on TCP or in Coll I overlay gels. In order to understand the mechanism of cluster formation we used several inhibitors to block receptors for rBM components laminin and collagen IV and found that both proteins were necessary for cell clustering. Surprisingly, we found that interaction with laminin through the 67LR, and not the laminin integrin α6β1, was critical for clustering. We next examined the impact of factors secreted by macrophages on these cells and determined that THP-1 conditioned media induced changes in cell morphology and proliferation, differences that were unique to cells cultured in rBM. Similar results were observed when cells were treated with conditioned media from differentiated HL-60, another macrophage cell model (Supplemental Figure 7). Finally, we examined the composition of THP-1 conditioned media and identified CXCL8 and HB-EGF as candidate stimuli that impact ectopic epithelial cells.
Previous studies have reported that culture with rBM can induce the formation of multi-cellular structures that appear to be more physiologically relevant than cells grown in monolayers on TCP. For example, mammary epithelial cells cultured in rBM gels form hollow acini that differentiate and secrete casein, while the same cells cultured as monolayers on TCP do not.35, 36 Similarly, primary epithelial cells isolated from the eutopic endometrium cultured on rBM inserts formed multi-cellular glands that remained responsive to hormonal stimulation.11, 37 To our knowledge, this is the first reconstruction of a multi-cellular model of ectopic endometriotic epithelial cells using rBM. When cultured in rBM gels, 12Z formed multi-cellular clusters similar to the multi-cellular structures seen in ectopic lesions in vivo.38 These lesions can remain relatively benign or become invasive, an event that may correlate to the development of severe pelvic pain.39 Interestingly, 12Z cells were previously reported to be invasive when cultured on rBM coated filters.12 While invasion into rBM was observed after long-term culture, treatment with THP-1 conditioned media, or treatment with HB-EGF (Supplemental Figure 1, Figures 7 and 9), significant invasion was not seen within the time frame previously reported for rBM coated filters (48 hours). Two important experimental variations may explain these differing observations. First, in the previous report,12 the 12Z cells were cultured on top of rBM while in this study cells were cultured using a 3D overlay of rBM. Culture in 3D ECM rather than on top of 2D ECM has been shown to impact morphology and gene expression for other cell types.40, 41 Secondly, cells were cultured at high densities in this study, resulting in the formation of cell-cell contacts, which can also alter cells such that they exhibit a less invasive phenotype.42
Using this multi-cellular model, we observed differences in the 12Z response to macrophage-derived factors compared to cells cultured on TCP (Figures 6 and 7). There are several variations between rBM and tissue culture plastic that could explain the heightened sensitivity to macrophage-derived factors observed for cells in rBM culture. First, the stiffness of the two materials is very different - tissue culture plastic has a modulus of approximately 109 Pa,43 while rBM gels are approximately 450 Pa.44 Numerous studies have demonstrated that material stiffness can impact sensitivity of cells to soluble stimuli45, 46 and influence cell behaviors such as adhesion, proliferation, migration, and differentiation.47 Secondly, the interactions between cells cultured in rBM gels were different than those of cells in a monolayer as cells in rBM form multi-cellular clusters (Figure 2). Maintenance of multi-cellular structures has been shown to impact cellular and tissue level functions.48 Finally, cells cultured in ECM gels interact with these gels through different integrins than cells cultured on TCP substrates, which could alter cellular response to growth factors. rBM is primarily composed of collagen IV and laminin, which are bound by α1β1, α10β1, α3β1, α6β1, α6β4, and the non-integrin 67 kDa laminin receptor while Coll I is bound by α1β1 and α2β1.49 Cells interact with TCP through adsorbed serum proteins such as vitronectin,50 which is bound by integrins such as αvβ1, αvβ3, and αvβ5.49 To clarify which difference was responsible for the increased sensitivity on rBM, 12Z cells were cultured in Coll I gels, which have similar stiffness to rBM gels (approximately 300 Pa51) but do not induce multi-cellular structures (Figure 2). Cells cultured in Coll I behaved similarly to cells cultured on TCP, indicating the increased sensitivity was not a result of stiffness changes alone and could be in response to differential integrin activation or the altered cell morphology. To explore the role of outside-in signaling from the ECM, cell receptors for collagen IV or laminin were blocked. When all receptors for collagen IV were blocked using soluble collagen IV, 12Z created long web-like patterns (Figure 5C) suggesting that collagen IV in the basement membrane may play a role in the contraction of cells into tightly packed clusters. Further, when 12Z were treated with the 67LR antagonist melibiose, but not with the anti-α6 integrin antibody, overall cluster size significantly decreased. Instead of forming large tight clusters, as in the vehicle control, 12Z formed small clusters comprised of fewer cells, suggesting that laminin plays a major role in cluster formation specifically through its interaction with the 67LR receptor (Figure 5D). Previous studies have shown that the 67LR is associated with increased invasiveness in many different cancers52, 53 and has been shown to increase cancer cell adhesion.54 Thus, it is possible that the 67LR mediates 12Z multicellular cluster formation by promoting cell motility.
To examine which factors from macrophage-conditioned media were responsible for the increase in cell proliferation on rBM, 12Z cells were treated with factors that were identified in the THP-1 conditioned media. Of the factors investigated, 12Z cells responded only to CXCL8 and HB-EGF. CXCL8 is a ligand for CXCR1 and CXCR2, and stimulates angiogenesis as well as proliferation and migration of cells.55 CXCL8 levels were reported to be elevated in endometriosis,30, 56 although the level of CXCL8 did not correlate with pain experienced by patients.57 Analysis of the role of CXCL8 in endometriosis has primarily focused on its impact on stromal cells, which produce CXCL8 that directs cell proliferation in an autocrine manner.58 12Z cells have previously been shown to produce CXCL8 when stimulated with TNFα,59 but the impact of CXCL8 on ectopic epithelial cell proliferation has not been reported. HB-EGF is a member of the epidermal growth factor (EGF)-like growth factor family that acts as a ligand for both the EGF receptor (ErbB1) and ErbB4. HB-EGF has been shown to activate proliferation through ErbB1 and chemotaxis and migration through ErbB4 in NIH 3T3 cells engineered to express only ErbB1 or ErbB4.60 HB-EGF has been shown to have increased expression prior to implantation in the apical surface of the luminal epithelium in the human endometrium.61 Further, increased expression of HB-EGF has been found in cytotrophoblasts and syncytiotrophoblasts of the chorionic villi early in pregnancy in humans and has been found to stimulate the invasion of embryonic trophoblasts into the surrounding epithelium during implantation in humans.62 To our knowledge, the effect of HB-EGF in endometriosis has not been reported. It is interesting to note that in contrast to CXCL8, HB-EGF treatment also led to increased invasion into the rBM. This is of particular interest as invasive and proliferative characteristics of endometriotic lesions differ based on the stage and severity of the disease.63 Thus, this data lends HB-EGF as a possible growth factor involved in the pathophysiology of endometriosis.
Combined, our results indicate that soluble factors secreted by macrophages impact ectopic epithelial cell morphology, proliferation, and invasiveness, and that these effects were observed when cells were cultured in a basement membrane context and not in a stromal context. These results suggest that in order to develop new therapeutic approaches to control endometriosis, it will be critical to develop physiologically-relevant in vitro models. Future studies using the rBM overlay culture system could incorporate additional cellular elements to more completely capture the in vivo physiology. For example, stromal cells make up a large portion of the ectopic lesion and are involved in the aberrant hormonal response seen in endometriosis.64 Additionally, the use of primary macrophages would allow for characterization of how in vitro lesions differ in response to M1 or M2 macrophages or macrophages from healthy women versus women with the disease.18
Supplementary Material
Acknowledgments
We would like to acknowledge Anthony Desotell and Danielle Bourgeois for help with the ErbB ligand ELISAs, Adriana Rodriguez for assistance with the proliferation experiments, Alex LaPerle for providing the ECM adsorption protocols, and the imaging assistance of the Laboratory for Optical and Computational Instrumentation (LOCI) at the University of Wisconsin-Madison. We gratefully acknowledge Dr. Kristyn Masters and Dr. Brenda Ogle for use of the time-lapse microscope. Funding for this work was provided by NSF CBET-0951613 (P.K.K.), American Cancer Society RSG-13-026-01-CSM (P.K.K.), UW-Madison Graduate Research School Grant (P.K.K.), and a NSF GRFP (M.J.C.).
Biography
Pamela Kreeger earned a BS in Chemistry from Valparaiso University and a PhD in Chemical Engineering at Northwestern University, where she was a NDSEG fellow. In her thesis work in the laboratories of Dr. Lonnie Shea and Dr. Teresa Woodruff, she developed a novel 3D culture system for ovarian follicles. She went on to an American Cancer Society post-doctoral fellowship in Dr. Doug Lauffenburger’s laboratory at MIT, where she utilized multivariate analysis tools to examine the impact of RAS mutations in colon cancer. Dr. Kreeger began as an Assistant Professor in Biomedical Engineering at the University of Wisconsin-Madison in 2009. Her lab utilizes tools from systems biology and tissue engineering to determine how the interactions between multiple components of the disease microenvironment influence cellular phenotypic decisions. She is the recipient of a NSF CAREER award, is an American Cancer Society Research Scholar, and was named a 2014 Emerging Investigator by Chemical Communications. Photo credit: David Nevala Photography.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to report.
References
- 1.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 2.Falcone T, Lebovic DI. Clinical management of endometriosis. Obstet Gynecol. 2011;118:691–705. doi: 10.1097/AOG.0b013e31822adfd1. [DOI] [PubMed] [Google Scholar]
- 3.Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol. 2007;213:565–573. doi: 10.1002/jcp.21237. [DOI] [PubMed] [Google Scholar]
- 4.Weaver VM, Fischer AH, Peterson OW, Bissell MJ. The importance of the microenvironment in breast cancer progression: recapitulation of mammary tumorigenesis using a unique human mammary epithelial cell model and a three-dimensional culture assay. Biochem Cell Biol. 1996;74:833–851. doi: 10.1139/o96-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 6.Beliard A, Donnez J, Nisolle M, Foidart JM. Localization of laminin, fibronectin, E-cadherin, and integrins in endometrium and endometriosis. Fertil Steril. 1997;67:266–272. doi: 10.1016/S0015-0282(97)81909-7. [DOI] [PubMed] [Google Scholar]
- 7.Harrington DJ, Lessey BA, Rai V, Bergqvist A, Kennedy S, Manek S, Barlow DH, Mardon HJ. Tenascin is differentially expressed in endometrium and endometriosis. J Pathol. 1999;187:242–248. doi: 10.1002/(SICI)1096-9896(199901)187:2<242::AID-PATH221>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 8.Aplin JD. Endometrial extracellular matrix. In: Aplin JD, Fazleabas AT, Glasser SR, Giudice LC, editors. The Endometrium: Molecular, Cellular, and Clinical Perspectives. 2. Boca Raton, FL: Informa UK Ltd; 2008. pp. 364–378. [Google Scholar]
- 9.Klemmt PA, Carver JG, Koninckx P, McVeigh EJ, Mardon HJ. Endometrial cells from women with endometriosis have increased adhesion and proliferative capacity in response to extracellular matrix components: towards a mechanistic model for endometriosis progression. Hum Reprod. 2007;22:3139–3147. doi: 10.1093/humrep/dem262. [DOI] [PubMed] [Google Scholar]
- 10.Bentin-Ley U, Pedersen B, Lindenberg S, Larsen JF, Hamberger L, Horn T. Isolation and culture of human endometrial cells in a three-dimensional culture system. J Reprod Fertil. 1994;101:327–332. doi: 10.1530/jrf.0.1010327. [DOI] [PubMed] [Google Scholar]
- 11.Arnold JT, Kaufman DG, Seppala M, Lessey BA. Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Hum Reprod. 2001;16:836–845. doi: 10.1093/humrep/16.5.836. [DOI] [PubMed] [Google Scholar]
- 12.Zeitvogel A, Baumann R, Starzinski-Powitz A. Identification of an invasive, N-cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am J Pathol. 2001;159:1839–1852. doi: 10.1016/S0002-9440(10)63030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kyama CM, Debrock S, Mwenda JM, D’Hooghe TM. Potential involvement of the immune system in the development of endometriosis. Reprod Biol Endocrinol. 2003;1:123. doi: 10.1186/1477-7827-1-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1–10. doi: 10.1016/s0015-0282(00)01630-7. [DOI] [PubMed] [Google Scholar]
- 15.Chacho KJ, Chacho MS, Andresen PJ, Scommegna A. Peritoneal fluid in patients with and without endometriosis: prostanoids and macrophages and their effect on the spermatozoa penetration assay. Am J Obstet Gynecol. 1986;154:1290–1299. doi: 10.1016/0002-9378(86)90715-5. [DOI] [PubMed] [Google Scholar]
- 16.Dunselman GA, Hendrix MG, Bouckaert PX, Evers JL. Functional aspects of peritoneal macrophages in endometriosis of women. J Reprod Fertil. 1988;82:707–710. doi: 10.1530/jrf.0.0820707. [DOI] [PubMed] [Google Scholar]
- 17.Wu MY, Ho HN. The role of cytokines in endometriosis. Am J Reprod Immunol. 2003;49:285–296. doi: 10.1034/j.1600-0897.2003.01207.x. [DOI] [PubMed] [Google Scholar]
- 18.Bacci M, Capobianco A, Monno A, Cottone L, Di Puppo F, Camisa B, Mariani M, Brignole C, Ponzoni M, Ferrari S, Panina-Bordignon P, Manfredi AA, et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol. 2009;175:547–556. doi: 10.2353/ajpath.2009.081011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loh FH, Bongso A, Fong CY, Koh DR, Lee SH, Zhao HQ. Effects of peritoneal macrophages from women with endometriosis on endometrial cellular proliferation in an in vitro coculture model. Fertil Steril. 1999;72:533–538. doi: 10.1016/s0015-0282(99)00292-7. [DOI] [PubMed] [Google Scholar]
- 20.Eyster KM, Hansen KA, Winterton E, Klinkova O, Drappeau D, Mark-Kappeler CJ. Reciprocal communication between endometrial stromal cells and macrophages. Reprod Sci. 2010;17:809–822. doi: 10.1177/1933719110371854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Starzinski-Powitz A, Guo SW. Constitutive and tumor necrosis factor-alpha-stimulated activation of nuclear factor-kappaB in immortalized endometriotic cells and their suppression by trichostatin A. Gynecol Obstet Invest. 2010;70:23–33. doi: 10.1159/000279324. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez KJ, Masters KS. Regulation of valvular interstitial cell calcification by components of the extracellular matrix. J Biomed Mater Res A. 2009;90:1043–1053. doi: 10.1002/jbm.a.32187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4:359–365. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuchiya S, Kobayashi Y, Goto Y, Okumura H, Nakae S, Konno T, Tada K. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 1982;42:1530–1536. [PubMed] [Google Scholar]
- 25.Yang J, Richards J, Bowman P, Guzman R, Enami J, McCormick K, Hamamoto S, Pitelka D, Nandi S. Sustained growth and three-dimensional organization of primary mammary tumor epithelial cells embedded in collagen gels. Proc Natl Acad Sci U S A. 1979;76:3401–3405. doi: 10.1073/pnas.76.7.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson PJ, Tatara A, McCreedy DA, Shiu A, Sakiyama-Elbert SE. Tissue-engineered fibrin scaffolds containing neural progenitors enhance functional recovery in a subacute model of SCI. Soft Matter. 2010;6:5127–5137. doi: 10.1039/c0sm00173b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muranen T, Selfors LM, Worster DT, Iwanicki MP, Song L, Morales FC, Gao S, Mills GB, Brugge JS. Inhibition of PI3K/mTOR leads to adaptive resistance in matrix-attached cancer cells. Cancer Cell. 2012;21:227–239. doi: 10.1016/j.ccr.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauvois B, Roth S. Initial adhesion of murine fibroblasts to collagen and fibronectin occurs by two mechanisms. Cell Biochem Funct. 1987;5:281–287. doi: 10.1002/cbf.290050407. [DOI] [PubMed] [Google Scholar]
- 29.Gu X, Masters KS. Regulation of valvular interstitial cell calcification by adhesive peptide sequences. J Biomed Mater Res A. 2010;93:1620–1630. doi: 10.1002/jbm.a.32660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malhotra N, Karmakar D, Tripathi V, Luthra K, Kumar S. Correlation of angiogenic cytokines-leptin and IL-8 in stage, type and presentation of endometriosis. Gynecol Endocrinol. 2011 doi: 10.3109/09513590.2011.593664. [DOI] [PubMed] [Google Scholar]
- 31.Lin W, Chen S, Li M, Wang B, Qu X, Zhang Y. Expression of macrophage migration inhibitory factor in human endometriosis: relation to disease stage, menstrual cycle and infertility. J Obstet Gynaecol Res. 2010;36:344–351. doi: 10.1111/j.1447-0756.2009.01123.x. [DOI] [PubMed] [Google Scholar]
- 32.Kondera-Anasz Z, Sikora J, Mielczarek-Palacz A, Jonca M. Concentrations of interleukin (IL)-1alpha, IL-1 soluble receptor type II (IL-1 sRII) and IL-1 receptor antagonist (IL-1 Ra) in the peritoneal fluid and serum of infertile women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2005;123:198–203. doi: 10.1016/j.ejogrb.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Miller MA, Meyer AS, Beste MT, Lasisi Z, Reddy S, Jeng KW, Chen CH, Han J, Isaacson K, Griffith LG, Lauffenburger DA. ADAM-10 and -17 regulate endometriotic cell migration via concerted ligand and receptor shedding feedback on kinase signaling. Proc Natl Acad Sci U S A. 2013;110:E2074–2083. doi: 10.1073/pnas.1222387110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li ML, Aggeler J, Farson DA, Hatier C, Hassell J, Bissell MJ. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci U S A. 1987;84:136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Classen-Linke I, Kusche M, Knauthe R, Beier HM. Establishment of a human endometrial cell culture system and characterization of its polarized hormone responsive epithelial cells. Cell Tissue Res. 1997;287:171–185. doi: 10.1007/s004410050743. [DOI] [PubMed] [Google Scholar]
- 38.Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–275. doi: 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- 39.Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11:595–606. doi: 10.1093/humupd/dmi029. [DOI] [PubMed] [Google Scholar]
- 40.Hong H, Stegemann JP. 2D and 3D collagen and fibrin biopolymers promote specific ECM and integrin gene expression by vascular smooth muscle cells. J Biomater Sci Polym Ed. 2008;19:1279–1293. doi: 10.1163/156856208786052380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grinnell F, Ho CH, Tamariz E, Lee DJ, Skuta G. Dendritic fibroblasts in three-dimensional collagen matrices. Mol Biol Cell. 2003;14:384–395. doi: 10.1091/mbc.E02-08-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 43.Callister WD. Fundamentals of Materials Science and Engineering: An Interactive E-Text. Somerset, NJ: John Wiley & Sons, Inc; 2000. [Google Scholar]
- 44.Soofi SS, Last JA, Liliensiek SJ, Nealey PF, Murphy CJ. The elastic modulus of Matrigel as determined by atomic force microscopy. J Struct Biol. 2009;167:216–219. doi: 10.1016/j.jsb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JH, Asthagiri AR. Matrix stiffening sensitizes epithelial cells to EGF and enables the loss of contact inhibition of proliferation. J Cell Sci. 2011;124:1280–1287. doi: 10.1242/jcs.078394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown XQ, Bartolak-Suki E, Williams C, Walker ML, Weaver VM, Wong JY. Effect of substrate stiffness and PDGF on the behavior of vascular smooth muscle cells: implications for atherosclerosis. J Cell Physiol. 2010;225:115–122. doi: 10.1002/jcp.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peyton SR, Ghajar CM, Khatiwala CB, Putnam AJ. The emergence of ECM mechanics and cytoskeletal tension as important regulators of cell function. Cell Biochem Biophys. 2007;47:300–320. doi: 10.1007/s12013-007-0004-y. [DOI] [PubMed] [Google Scholar]
- 48.Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27:714–723. doi: 10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lowell CA, Mayadas TN. Overview: studying integrins in vivo. Methods Mol Biol. 2012;757:369–397. doi: 10.1007/978-1-61779-166-6_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayman EG, Pierschbacher MD, Suzuki S, Ruoslahti E. Vitronectin--a major cell attachment-promoting protein in fetal bovine serum. Exp Cell Res. 1985;160:245–258. doi: 10.1016/0014-4827(85)90173-9. [DOI] [PubMed] [Google Scholar]
- 51.Raub CB, Putnam AJ, Tromberg BJ, George SC. Predicting bulk mechanical properties of cellularized collagen gels using multiphoton microscopy. Acta Biomater. 2010;6:4657–4665. doi: 10.1016/j.actbio.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song T, Choi CH, Cho YJ, Sung CO, Song SY, Kim TJ, Bae DS, Lee JW, Kim BG. Expression of 67-kDa laminin receptor was associated with tumor progression and poor prognosis in epithelial ovarian cancer. Gynecol Oncol. 2012;125:427–432. doi: 10.1016/j.ygyno.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 53.Li D, Chen J, Gao Z, Li X, Yan X, Xiong Y, Wang S. 67-kDa laminin receptor in human bile duct carcinoma. Eur Surg Res. 2009;42:168–173. doi: 10.1159/000198234. [DOI] [PubMed] [Google Scholar]
- 54.Chetty C, Khumalo T, Da Costa Dias B, Reusch U, Knackmuss S, Little M, Weiss SF. Anti-LRP/LR Specific Antibody IgG1-iS18 Impedes Adhesion and Invasion of Liver Cancer Cells. PLoS One. 2014;9:e96268. doi: 10.1371/journal.pone.0096268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 56.Bersinger NA, von Roten S, Wunder DM, Raio L, Dreher E, Mueller MD. PAPP-A and osteoprotegerin, together with interleukin-8 and RANTES, are elevated in the peritoneal fluid of women with endometriosis. Am J Obstet Gynecol. 2006;195:103–108. doi: 10.1016/j.ajog.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 57.Scholl B, Bersinger NA, Kuhn A, Mueller MD. Correlation between symptoms of pain and peritoneal fluid inflammatory cytokine concentrations in endometriosis. Gynecol Endocrinol. 2009;25:701–706. doi: 10.3109/09513590903159680. [DOI] [PubMed] [Google Scholar]
- 58.Li MQ, Luo XZ, Meng YH, Mei J, Zhu XY, Jin LP, Li DJ. CXCL8 enhances proliferation and growth and reduces apoptosis in endometrial stromal cells in an autocrine manner via a CXCR1-triggered PTEN/AKT signal pathway. Hum Reprod. 2012;27:2107–2116. doi: 10.1093/humrep/des132. [DOI] [PubMed] [Google Scholar]
- 59.Grund EM, Kagan D, Tran CA, Zeitvogel A, Starzinski-Powitz A, Nataraja S, Palmer SS. Tumor necrosis factor-alpha regulates inflammatory and mesenchymal responses via mitogen-activated protein kinase kinase, p38, and nuclear factor kappaB in human endometriotic epithelial cells. Mol Pharmacol. 2008;73:1394–1404. doi: 10.1124/mol.107.042176. [DOI] [PubMed] [Google Scholar]
- 60.Elenius K, Paul S, Allison G, Sun J, Klagsbrun M. Activation of HER4 by heparin-binding EGF-like growth factor stimulates chemotaxis but not proliferation. EMBO J. 1997;16:1268–1278. doi: 10.1093/emboj/16.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoo HJ, Barlow DH, Mardon HJ. Temporal and spatial regulation of expression of heparin-binding epidermal growth factor-like growth factor in the human endometrium: a possible role in blastocyst implantation. Dev Genet. 1997;21:102–108. doi: 10.1002/(SICI)1520-6408(1997)21:1<102::AID-DVG12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 62.Leach RE, Khalifa R, Ramirez ND, Das SK, Wang J, Dey SK, Romero R, Armant DR. Multiple roles for heparin-binding epidermal growth factor-like growth factor are suggested by its cell-specific expression during the human endometrial cycle and early placentation. J Clin Endocrinol Metab. 1999;84:3355–3363. doi: 10.1210/jcem.84.9.5980. [DOI] [PubMed] [Google Scholar]
- 63.Matsuzaki S, Darcha C. Epithelial to mesenchymal transition-like and mesenchymal to epithelial transition-like processes might be involved in the pathogenesis of pelvic endometriosis. Hum Reprod. 2012;27:712–721. doi: 10.1093/humrep/der442. [DOI] [PubMed] [Google Scholar]
- 64.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.