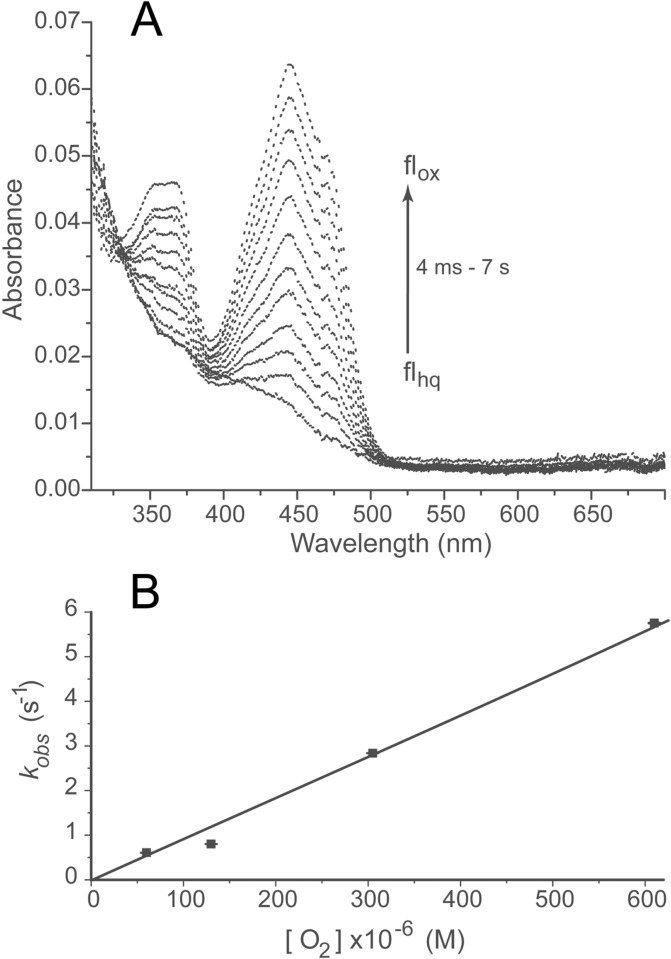
Figure 4.
Oxidation of hIYD·flhq by O2. A solution containing hIYD·flhq (5 μM final) in 500 mM NaCl, 10% glycerol, 1 mM DTT, and 50 mM sodium phosphate, pH 7.4, was mixed with an equal volume of an oxygenated solution containing 500 mM NaCl, 10% glycerol, 1 mM DTT, and 50 mM sodium phosphate, pH 7.4. (A) Spectra of hIYD·flhq oxidation by air-saturated buffer were recorded from 4 ms to 7 s with a diode array spectrophotometer. The arrow indicates the direction of spectral change as a function of time. (B) Oxidation of hIYD·flhq was monitored by absorbance at 446 nm, and the resulting kobs values (see Figure S3) were plotted against oxygen concentration to determine the second-order rate constant (kox). Data points represent the average of three independent measurements, and the standard deviations are illustrated by error bars. The solid line was generated by a linear best fit to the data.