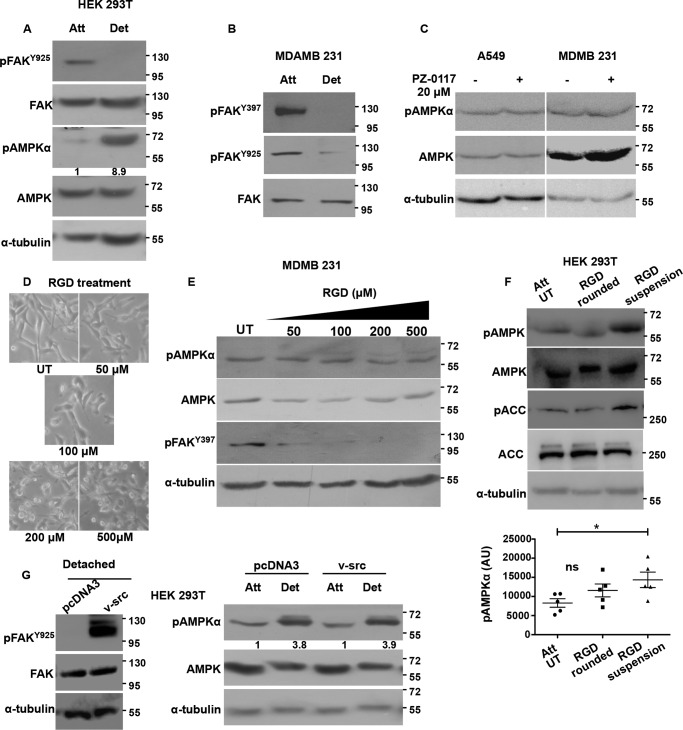
FIGURE 2.
AMPK phosphorylation is independent of FAK and Src signaling. HEK 293T cells (A) and MDA-MB 231 cells (B) were cultured under attached (Att) and detached (Det) (10 min) conditions, and levels of indicated total proteins and phosphoproteins were determined by Western blotting (n = 3). C, A549 and MDA-MB 231 cells were cultured under attached conditions and treated for 1 h with FAK inhibitor PZ-0117 (20 μm). pAMPKα and AMPK levels were measured by Western blotting (n = 3). D, MDA-MB 231 cells cultured under attached conditions were treated with RGD peptide at the concentrations specified for 2 h. Phase contrast images for cell morphology changes were taken at 2 h post-treatment with RGD, and E, cells were lysed and pAMPKα, AMPK, and pFAKTyr-397 levels were measured by Western blotting (n = 5). F, HEK 293T cells were treated with RGD (200 μm) for 10 min (leading to cell rounding) or 30 min (leading to cell detachment). Cells were lysed, and pAMPKα, pACC, total AMPK, and ACC were determined by Western blotting (n = 5). The scatterplot depicts raw pAMPK densitometric values normalized to the relative tubulin levels in each experiment .*, p < 0.05. Error bars represent ± S.E. G, HEK 293T cells were transfected with vector control pcDNA3 or constitutively active v-Src construct. After 24 h, the transfected cells were then cultured in attached or detached conditions. Phosphorylation of FAK by v-Src at Tyr-925 was determined by Western blotting under detached conditions. Levels of pAMPKα and AMPK were probed by Western blotting under attached and detached conditions (n = 3). Numbers depict relative pAMPKα/AMPK ratio. ns, non-significant; AU, arbitrary units; UT, were treated with vehicle.