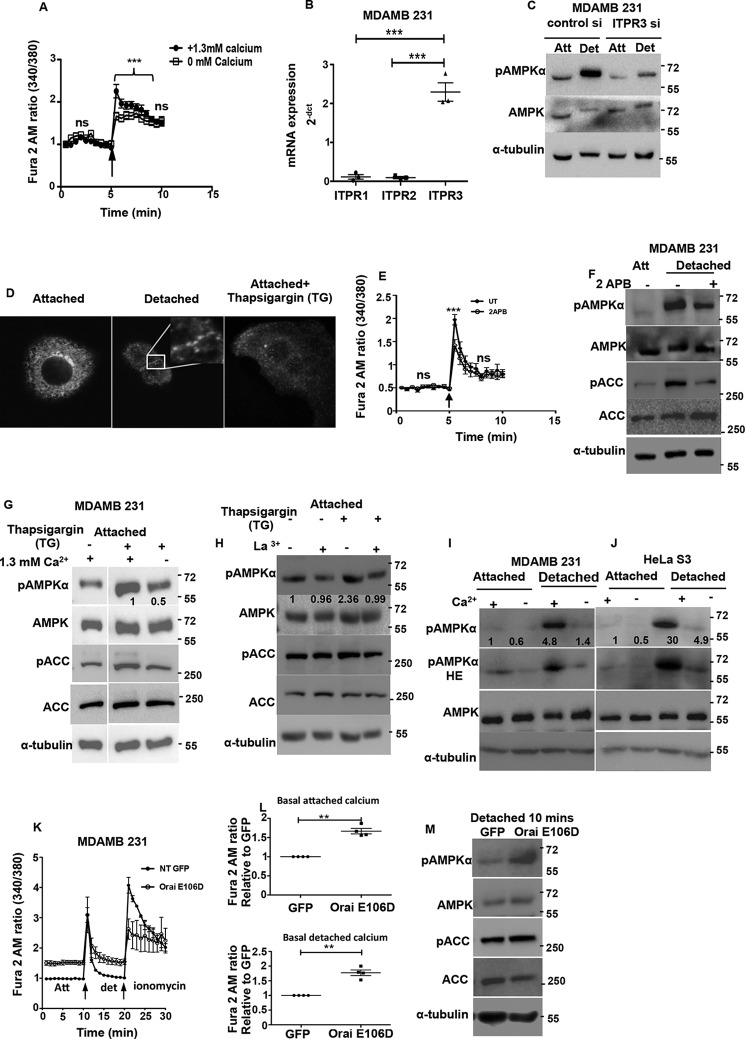
FIGURE 6.
ER calcium release and store-operated calcium entry contributes to AMPK activation upon matrix deprivation. A, MDA-MB 231 cells were loaded with Fura 2 AM and cultured in calcium-containing or calcium-free buffer. Graph depicts time course measurements of emission obtained at 510 nm by excitation of the dye at 340 and 380 nm (340:380 ratio). The black arrow represents the point of mechanical detachment in the time course. Values have been normalized to the initial reading. Values are expressed as mean ± S.E.; ***, p < 0.001 (n = 4 with three technical repeats each). ns, non-significant. B, scatterplot depicts 2−dct values representing the mRNA expression of ITPR receptor subtypes determined by qRT-PCR (n = 3 biological samples with three technical repeats each). C, MDA-MB 231 cells were transfected with either control siRNA or ITPR3 siRNA and cultured under attached (Att) and detached (Det) conditions. Cell lysates were probed by Western blotting with the antibodies indicated. D, MCF7 cells were transfected with STIM1 YFP construct and cultured under attached and detached conditions. Representative confocal equatorial sections are shown. Thapsigargin (TG) was used as positive control. E, MDA-MB 231 cells were loaded with Fura 2 AM and pre-treated with either vehicle control (UT) or SOCE inhibitor 2-APB at 50 μm for 10 min. Graph depicts time course measurements of emission obtained at 510 nm by excitation of the dye at 340 and 380 nm (340:380 ratio). The black arrow represents the point of mechanical detachment in the time course. Values have been normalized to the initial reading. Values are expressed as mean ± S.E.; ***, p < 0.05 (n = 4 with three technical repeats each). F, MDA-MB 231 cells were cultured under attached conditions and pretreated with 100 μm 2-APB for 10 min prior to detachment. Cell lysates were subjected to Western blotting with the antibodies indicated (n = 3). G, attached MDA-MB 231 cells were treated with vehicle control or thapsigargin (TG) (200 nm) for 10 min in the presence or absence of extracellular calcium as indicated. Lysates were run together in the same gel and subjected to immunoblotting with the antibodies indicated (n = 3). H, attached MDA-MB 231 cells were treated with vehicle control or thapsigargin (200 nm) for 10 min in the presence or absence of La3+ (10 μm). Cell lysates were subjected to Western blotting for the antibodies indicated (n = 3). I, MDA-MB 231 cells were cultured in calcium-containing or calcium-free conditions for 30 min and detached as indicated for 10 min. Lysates were subjected to immunoblotting with the antibodies indicated (n = 3). Numbers depict relative pAMPKα/tubulin ratio. J, HeLa S3 cells were cultured in calcium-containing or calcium-free conditions for 30 min and detached as indicated for 10 min. Lysates were subjected to immunoblotting with the antibodies indicated (n = 3). Numbers depict relative pAMPKα/tubulin ratio. K and L, MDA-MB 231-GFP and MDA-MB 231 Orai E106D GFP cells were loaded with Fura 2 AM and cultured in calcium-containing KH buffer. Graph depicts time course measurements of emission obtained at 510 nm by excitation of the dye at 340 and 380 nm (340:380 ratio). The black arrow represents the point of mechanical detachment and ionomycin addition in the time course. Values are expressed as mean ± S.E.; **, p < 0.01 (n = 4 with 5 technical repeats each). M, MDA-MB 21 GFP and Orai E106D GFP cells were lysed under detached conditions, and lysates were subjected to Western blotting with the antibodies indicated.