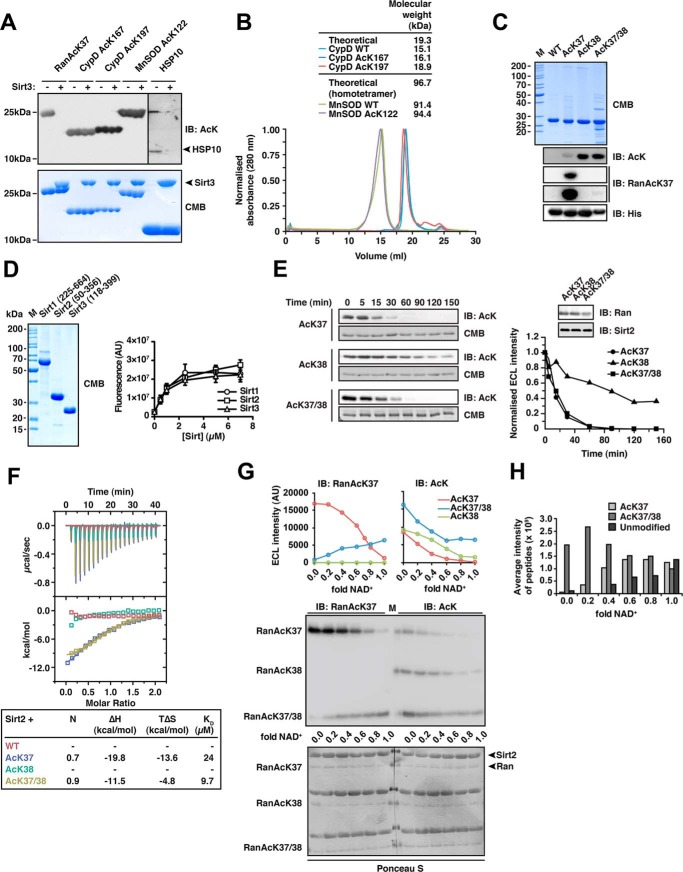
FIGURE 1.
Sirt2 can deacetylate di-acetylated Ran. A, deacetylation of selected substrate proteins by Sirt3. We prepared site-specifically acetylated Ran AcK37, CypD AcK167, and AcK197, MnSOD AcK122, and Hsp10 AcK56 using the genetic code expansion concept and analyzed its deacetylation by Sirt3 by immunoblotting using an anti-acetyl-lysine antibody (anti-AcK-AB). Sirt3 was used in equimolar ratio with the respective protein substrate (40 μm), except for Hsp10 (40 μm Sirt3 and 80 μm Hsp10). Coomassie Brilliant (CMB) blue staining is shown as loading control. B, analytical size exclusion chromatography of CypD and MnSOD proteins used in this study (Superdex 200 10/300). To assess the quality of the non-acetylated and site-specifically lysine-acetylated CypD (WT, AcK167, and AcK197) and MnSOD (WT and MnSOD AcK122) proteins used here, we performed analytical SEC experiments. All CypD proteins eluted as apparent monomer from the SEC column. MnSOD behaved as apparent tetramer on SEC, as reported earlier. For both, MnSOD and CypD, all acetylated proteins behaved as the non-acetylated proteins showing that lysine acetylation did not affect protein folding or oligomerization. C, Ran was lysine-acetylated at Lys-38 and at Lys-37/Lys-38 using the genetic code expansion concept. Coomassie Brilliant Blue staining of an SDS-PAGE with 5 μg of protein shows the final purity. Anti-AcK-AB was used to show the successful incorporation of acetyl-l-lysine into Ran. The anti-AcK-AB detects acetyl-l-lysine with different sensitivity depending on the sequence context. Detection with anti-His6-AB serves as a loading control. An antibody raised against a Ran AcK37-derived 11-mer peptide (see “Experimental Procedures”) is specific for Ran AcK37, showing only a very weak signal for RanAcK37/38 after long exposure to x-ray film and does not detect Ran AcK38. D, final purity of the Sirt1, Sirt2, and Sirt3 enzymes used in this study. 5 μg of each sirtuin was separated by SDS-PAGE and the gel Coomassie Brilliant Blue-stained to assess the final purity. The enzymatic activities were tested with a fluor-de-lys assay. All enzymes are active and show a similar activity. Error bars represent the standard error of the mean for three independent measurements. E, kinetics of Ran AcK37, AcK38, and AcK37/38 deacetylation by Sirt2. 12 μm site-specifically lysine-acetylated Ran protein was incubated with 0.14 μm Sirt2 for the indicated times. The level of Ran acetylation was assessed by immunoblotting using a specific anti-AcK-AB (left). Ran AcK37 and di-acetylated Ran AcK37/38 were deacetylated with similar kinetics, whereas Ran AcK38 shows a strongly reduced Sirt2-catalyzed deacetylation rate. The SDS-polyacrylamide gels corresponding to the Western blots were stained with Coomassie Brilliant Blue and serve as a loading control. The reactions were quantified densitometrically using ImageJ software (right). F, Ran AcK37 and AcK37/38 bind to Sirt2 as shown by isothermal titration calorimetry. 45 μm Ran-WT, Ran AcK37, AcK38, or AcK37/38 protein was titrated with 450 μm Sirt2 (50–356, non-His6-tagged). Ran AcK37 and RanAcK37/38 bind with affinities of 24 and 9.7 μm, respectively. Both reactions are solely driven by the reaction enthalpy (Ran AcK37 ΔH, −19.8 kcal/mol; TΔS, −13.6 kcal/mol; Ran AcK37/38 ΔH,−11.5 kcal/mol; TΔS, −4.8 kcal/mol). No binding heat was observed under these conditions for Ran-WT and Ran AcK38. G, deacetylation of Ran AcK38 by Sirt2 is favored over AcK37 in di-acetylated Ran AcK37/38.12 μm Ran AcK37. AcK38 and AcK37/38 were used in Sirt2-mediated deacetylation reactions with increasing amounts of the essential cofactor NAD+ (Sirt2 concentration, 24 μm, molar ratio Ran AcK/NAD+, 0–1.0). Using the anti-Ran AcK37 AB, we observed a linear decrease of acetylated Ran AcK37. In contrast, the signal increased for di-acetylated AcK37/38 as a function of the NAD+ concentration. This suggests that AcK38 in the context of Ran AcK37/38 is deacetylated preferentially by Sirt2. As expected, AcK38 shows no signal using the anti-Ran AcK37 AB. Using the pan-anti-AcK-AB, all acetylated Ran proteins showed a linear decrease in the acetylation level. However, although AcK37 and AcK38 were nearly completely deacetylated at a molar Ran AcK/Sirt2 ratio of 1:1, this was not the case for di-acetylated AcK37/38R. Detection with an anti-Ran-AB was used as a loading control. Densitometric analyses of the signals obtained with the anti-AcK-AB and anti-Ran AcK37 are shown at the top. H, mass spectrometric analysis of the experiment is shown in F. The reactions were analyzed by digest with Glu-C followed by ESI-MS/MS. Without NAD+, there is only di-acetylated Ran present. With increasing NAD+ concentrations, the average intensity of peptides corresponding to di-acetylated Ran decreases and that of the non-acetylated Ran increases. The amount of Ran AcK37 peptide increases with increasing NAD+ concentrations suggesting that Ran AcK38 is deacetylated first. IB, immunoblot.