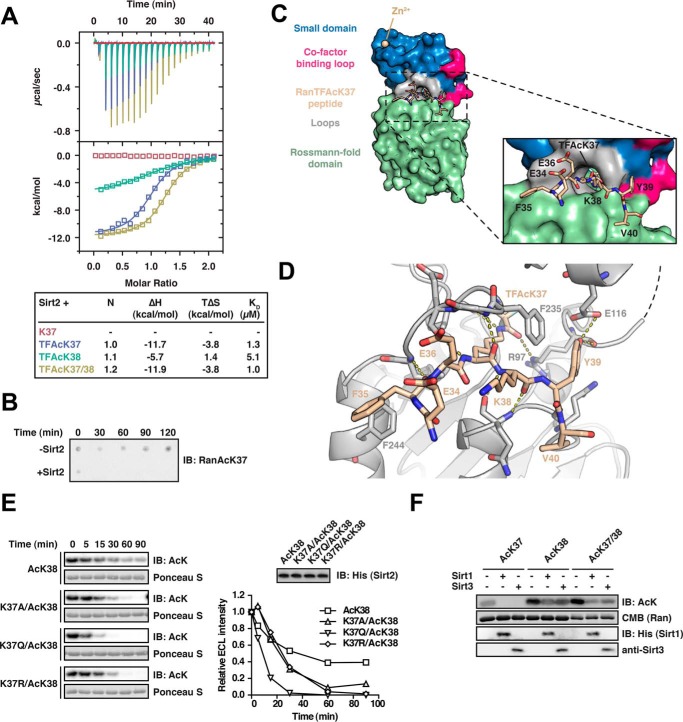
FIGURE 2.
Characterization of the Ran-Sirt2 interaction using trifluoroacetyl-lysine peptides and Ran mutants. A, Ran TFAcK37, TFAcK38, and TFAcK37/38 13-mer peptides bind to Sirt2 in the micromolar range as shown by isothermal titration calorimetry. 30 μm Sirt2 (50–356, non-His6-tagged) was titrated with 300 μm trifluorolysine-acetylated Ran 13-mer peptide (Ran amino acids 31–43). The reactions are all driven by a favorable reaction enthalpy, ΔH, whereas the reaction of the TFAcK38 peptide is the only one driven by both favorable enthalpy and favorable entropy, TΔS. This shows that the TFAcK38 peptide uses a distinct mechanism of binding. B, Ran 13-mer AcK37 peptide is deacetylated by Sirt2. 133 μm of Ran AcK37 13-mer peptide (Ran amino acids 31–43) was spotted on a nitrocellulose membrane. Addition of 0.5 μm Sirt2 leads to a complete deacetylation already after 30 min, compared with the non-enzyme control (−Sirt2). The specific anti-Ran AcK37 AB was used for detection by immunoblotting. C, crystal structure of Sirt2 (amino acids 50–356) in complex with the TFAcK37 13-mer Ran peptide (amino acids 31–43). The structure was solved at a resolution of 3 Å. Left, overall Sirt2·Ran(31–43) TFAcK37 structure. Sirt2 is shown as a surface representation. Blue, small Zn2+-binding domain; red, cofactor binding loop; green, Rossmann-fold domain; beige, Ran(31–43) TFAcK37 peptide. Right, close-up of the Ran(31–43) TFAcK37 peptide and Sirt2. Three charged residues Glu-34R, Glu-36R, and the Lys-38R are solvent-exposed. The hydrophobic tunnel leading to the Sirt2 active site is too narrow to simultaneously adopt two acetylated lysine residues. The TFAcK37 penetrates into a hydrophobic tunnel formed by the small subdomain and the Rossmann-fold domain. D, two aromatic residues Phe-35R and Tyr-39R of the Ran(31–43) 13-mer TFAcK37 peptide act as anchor points through the formation of stacking interactions with the aromatic residues in Phe-244 and Phe-235 of Sirt2, respectively. Furthermore, the peptide forms several main chain hydrogen bonds with Sirt2, highlighted with the dotted yellow lines, and the hydroxyl group of Tyr-39R forms a hydrogen bond with the main chain carbonyl-oxygen of Arg-97 of Sirt2. The peptide adopts a crescent- and w-shaped conformation. E, effects of Ran mutations on the kinetics of Sirt2-mediated di-deacetylation of Ran AcK38. To assess whether electrostatic or steric properties of AcK37R increase the deacetylation rate of AcK38R, we created Lys-37R mutant proteins in the Ran AcK38 background. The non-mutated mono-acetylated Ran AcK38 shows the slowest Sirt2-catalyzed deacetylation of the proteins compared. All mutant variants (RanK37A/AcK38, K37R/AcK38, and K37Q/AcK38) show an accelerated Sirt2-catalyzed deacetylation compared with the mono-acetylated Ran AcK38 protein (12 μm Ran and 0.06 μm Sirt2). This shows that both electrostatic and steric properties of AcK37R contribute to the observed accelerated Sirt2-mediated deacetylation of AcK38R. F, Sirt1 and Sirt3 are able to di-deacetylate Ran AcK37/38. Ran AcK37, Ran AcK38, and di-acetylated Ran AcK37/38 were incubated with Sirt1 or Sirt3, and the acetylation level was assessed by immunoblotting (IB) using an anti-AcK antibody (12 μm Ran was incubated with 0.8 μm of the indicated Sirtuin for 30 min). Ran AcK38 shows a relatively strong remaining anti-AcK signal compared with the di-acetylated AcK37/38R, suggesting that, as observed for Sirt2, the presence of AcK37R stimulates the deacetylation of AcK38R by Sirt1 and Sirt3. Coomassie (CMB) staining is shown as loading control for Ran. Sirtuins were detected with the indicated antibodies.