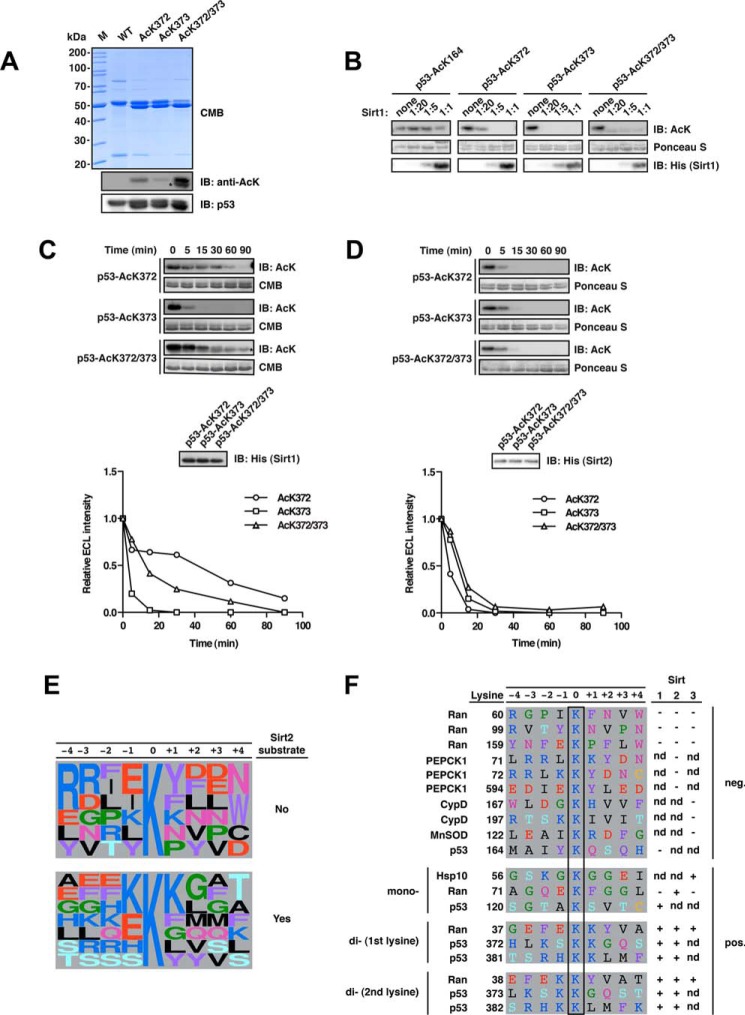
FIGURE 5.
Deacetylation of p53 at Lys-372 is enhanced upon acetylation of the neighboring Lys-373 for Sirt1 but not Sirt2. A, final purity and quality of p53-WT and acetylated p53 (AcK372, AcK373, and AcK372/372). For details on the experimental procedures see Fig. 1A. Notably, for p53 AcK372, AcK373, AcK381, AcK382, and the di-acetylated proteins, we obtained acetylated truncation products, which we could not remove during purification due to the similar size of the truncation product and the full-length protein and possibly due to oligomerization of the p53 protein. An asterisk denotes the anti-AcK-immunoreactive truncation product. B, p53 deacetylation by Sirt1 at AcK164, AcK372, AcK373, and AcK372/373 was analyzed at increasing molar Sirt1/p53 ratios (p53, 12 μm). The immunoblot with an anti-AcK-antibody shows that, at a molar ratio of Sirt1/p52 of 1:20, the weakest p53 deacetylation by Sirt1 occurs at AcK372, supporting the mechanism of di-deacetylation, by which the presence of AcK373 accelerates deacetylation at AcK372. p53-AcK164 is not deacetylated by Sirt1 in vitro even at a substrate/enzyme ratio of 1:1. C, time course of the Sirt1-catalyzed p53 AcK372, AcK373, and AcK372/373 deacetylation. p53 AcK373 is deacetylated by Sirt1 showing complete deacetylation after 30 min, whereas deacetylation is not completed for AcK372 after 90 min. The di-acetylated p53 AcK372/373 is deacetylated faster than p53 AcK372 (p53, 12 μm; Sirt1, 0.24 μm; molar ratio 1:50). The densitometric quantification was done using ImageJ software. The signal originating from the truncation product remains constant and was subtracted from the signal of the full-length p53 AcK372/373 (the band of the truncated fragment is denoted with an asterisk and runs slightly lower). The acetylation level was assessed using an anti-AcK-antibody. Coomassie Brilliant Blue (CMB) staining was used as a loading control for p53 and an anti-His6 antibody to detect Sirt1. D, time course of Sirt2-catalyzed deacetylation of p53 AcK372, AcK373, and AcK372/373 as described in C but with an enzyme/substrate ratio of 1:200 (0.06 μm Sirt1). All acetylated p53 variants show highly similar deacetylation kinetics with Sirt2. E, frequency plot of sequence context of lysine acetylation sites found in this and our previous study to be substrates or non-substrates of Sirt2 as indicated (14). Representation was created with WebLogo (106). F, sequence alignment of primary sequences surrounding di-acetylation motifs analyzed in this study and in our previous study (14). We discovered that several di-acetylation sites were deacetylated by Sirt1, Sirt2, and/or Sirt3 (Ran AcK37/38, p53 AcK372/373, and p53 AcK381/382). Interestingly, PEPCK1, although also containing a possible di-acetylation motif (AcK70/71) followed by a tyrosine residue, is not deacetylated by Sirt2, suggesting that the structure is an important denominator of Sirt specificity. Notably, the residues 67RRL69 in PEPCK1 preceding Lys-70/Lys-71 form a β-strand as part of an antiparallel β-sheet. It needs further investigation whether the replacement of this sequence by the corresponding Ran sequence 34EFE36 confers Sirt2 activity or whether this is due to structural effects interfering with the formation of this β-sheet, maybe affecting the loops flexibility. IB, immunoblot.