Abstract
Inherited retinal dystrophies (IRDs) are Mendelian diseases with tremendous genetic and phenotypic heterogeneity. Identification of the underlying genetic basis of these dystrophies is therefore challenging. In this study we employed whole exome sequencing (WES) in 11 families with IRDs and identified disease-causing variants in 8 of them. Sequence analysis of about 250 IRD-associated genes revealed 3 previously reported disease-associated variants in RHO, BEST1 and RP1. We further identified 5 novel pathogenic variants in RPGRIP1 (p.Ser964Profs*37), PRPF8 (p.Tyr2334Leufs*51), CDHR1 (p.Pro133Arg and c.439-17G>A) and PRPF31 (p.Glu183_Met193dup). In addition to confirming the power of WES in genetic diagnosis of IRDs, we document challenges in data analysis and show cases where the underlying genetic causes of IRDs were missed by WES and required additional techniques. For example, the mutation c.439-17G>A in CDHR1 would be rated unlikely applying the standard WES analysis. Only transcript analysis in patient fibroblasts confirmed the pathogenic nature of this variant that affected splicing of CDHR1 by activating a cryptic splice-acceptor site. In another example, a 33-base pair duplication in PRPF31 missed by WES could be identified only via targeted analysis by Sanger sequencing. We discuss the advantages and challenges of using WES to identify mutations in heterogeneous diseases like IRDs.
Introduction
Inherited retinal dystrophies (IRDs) are a group of rare but highly heterogeneous genetic disorders characterized by an abnormal function or degeneration of specific cell types in the retina, as for example photoreceptors. Consequently, partial or complete vision loss is experienced by affected individuals. These diseases are heterogeneous, not only in terms of age of onset, severity and progression of the disease, but also in terms of their underlying genetics[1]. Currently, there are around 250 genes, mutations in which have been reported to cause various forms of retinal dystrophies. These mutations can be inherited in an autosomal recessive, dominant or X-linked manner. Based on cells that are affected first during disease-progression, these diseases are also classified as either rod-dominated (e.g. retinitis pigmentosa, RP) or cone-dominated (e.g., cone-rod dystrophy, CORD). Moreover, mutations in the same gene have been shown to lead to variable phenotypes, adding to the already existing complexity.
Whole exome sequencing (WES) is an efficient method to identify disease-causing mutations, particularly for monogenic inherited disorders such as IRDs[2–4]. Although fast and accurate, WES fails to identify disease-causing mutations in almost 35% of the cases (Tiwari et al, unpublished data). Possible reasons include (i) variants in genes not yet disease-associated, (ii) variants that lie within deep intronic regions and are therefore missed by the exome capture methods, or (iii) limitations of the employed method that prevent efficient identification of sequence alterations. Complementary methods, e.g. autozygosity mapping or whole genome sequencing may be considered to facilitate the identification of the disease-associated genetic alterations.
General diagnostic approaches, applied in majority of the genetic laboratories, include Sanger sequencing of most frequently disease-associated gene(s), followed by either panel or whole exome sequencing. In this study, the majority of cases were first screened by Sanger sequencing for variants in most likely candidate genes. They were then subjected to whole exome sequencing. Initial analysis was focused to identify variants within 250 genes associated with different forms of retinal dystrophies. Additional family members were also recruited to perform segregation analysis of the mutation with the disease phenotype. We present examples of cases that highlight the challenges and limitations of WES data analysis, which could have implication towards procedures used to identify mutations in gene diagnostics and research projects.
Materials and Methods
Ethics Statement
The study was conducted in accordance to the Helsinki Declaration and carried out according to the approved protocols at University of Zürich as per Swissmedic guidelines. The approval for genetic testing in the frame of this study was awarded to the Institute of Medical Molecular Genetics by the Federal Office of Public Health (FOPH) in Switzerland.
Patients and families
Patients and family members were referred to us for genetic testing purposes from different eye clinics. All patients or family members as well as parents of affected children provided written informed consent for genetic testing. Pedigrees were drawn using PED6 software (http://www.medgen.de/ped/index.html). Information regarding family history, visual complaints and inheritance patterns of the diseases were collected through a standard ophthalmologic examination. All family members with a 5-digit patient ID represented in the pedigrees were included in this study. Family members not marked with a 5-digit ID did not participate in this study and no samples were analyzed.
DNA extraction
Venous blood extracted from patients was used to isolate genomic DNA in duplicate using a coated magnetic bead technology according to the manufacturer’s recommendations (PerkinElmer Chemagen Technologie GmbH, Baesweiler, Germany). DNA integrity was verified using the Nanodrop (Life technologies, Darmstadt, Germany).
Whole exome sequencing (WES) analysis
WES was performed at the Cologne Center for Genomics, University of Cologne, using NimbleGen SeqCap EZ Human Exome Library (Roche NimbleGen Inc., Madison, WI) for library preparation. Paired-end 100nt sequencing was performed on Illumina HiSeq2000. Alignment of sequence reads, indexing of the reference genome, variant calling and annotation was achieved using a pipeline based on BWA[5], Samtools[6], Picard (http://broadinstitute.github.io/picard/) and Annovar[7] respectively. Variants were annotated using Alamut-HT (Interactive Biosoftware, Rouen, France) and visualized on Alamut Viewer 2.2 (Interactive Biosoftware, Rouen, France). A filtering pipeline was established to remove known and frequent SNPs or benign polymorphisms. Variants with frequency less than 1% in the population were selected. Variants that have been described in literature and the Human Gene Mutation Database (HGMD) to be disease-associated were given higher priority. Within the variant types, protein truncation mutations leading to loss of function such as nonsense or frameshift mutations were given higher priority. Pathogenicity of missense variants were checked by five protein prediction algorithms SIFT[8], PolyPhen2[9], MutationTaster2[10], MAPP[11] and Align GVGD[12, 13].
Primer design, PCR amplification and Sanger sequencing
Most likely disease-causing variants were confirmed by Sanger sequencing in the patient and the available family members. Primers were designed using Primer3 algorithm[14] and purchased at Microsynth AG (Balgach, Switzerland). All target regions were amplified in duplicate from genomic DNA of the patients and available family members using Hot FirePol® DNA Polymerase (Solis BioDyne, Tartu, Estonia). PCR products were purified by treating them with ExoSAP reagent (Affymetrix, Santa Clara, CA) and sequenced using the Big Dye Terminator Cycle v1.1 Sequencing Kit (Applied Biosystems, Carlsbad, California, USA) and ABI Prism 3730 Genetic Analyzer (Applied Biosystems, Carlsbad, California, USA). Sanger sequencing data analysis was performed using the Sequencing Analysis Software v5.4, SeqScape v2.6 (Applied Biosystems, Carlsbad, California, USA), MutationSurveyorV5.0.0 (Soft Genetics, Pennsylvania, USA) and Chromas (Technelysium, South Brisbane, Australia) to identify the likely disease causing mutations. Mutation is defined as previously described[15].
Cell culture and splicing assay
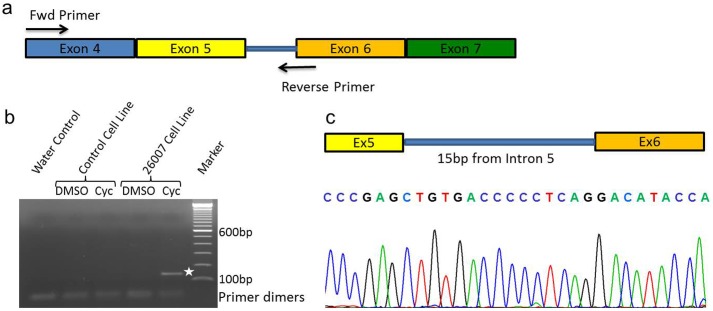
Patient derived fibroblasts were established as previously described[16, 17]. Cells were cultured in MEM medium substituted with 10% fetal calf serum, 1.3% L-glutamine, 0.8% antibiotic and antimycotic solution and incubated at 37°C and 5% CO2. 80% confluent cells were treated with 100μg/ml cycloheximide and incubated for 4 hours, upon which cells were harvested and total RNA was extracted using Qiagen RNeasy mini kit (Hombrechtikon, Switzerland). cDNA was generated from total RNA by reverse transcription with random primers according to manufacturer’s instructions (Supercript III, Invitrogen, Basel, Switzerland). Primers overlapping the intronic region (Intron 5 of CDHR1 gene) were designed in order to amplify a specific product in case of activation of the cryptic splice site in the patient cell line. RT-PCR reaction was performed and PCR products were analyzed on agarose gel. RT-PCR products were verified by sequencing.
Structural analysis
Structural modeling of CDHR1 (reference sequence NP_149091.1), PRPF31 (reference sequence NP_056444.3) and the respective mutant protein sequences was performed at iTasser server[18]. Visualization of the generated structures and their alignments were performed using PyMol (The PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC).
Results
Out of 11 cases diagnosed with either RP or CORD, we identified putative disease-associated variants in 8 cases. The clinical phenotypes of the patients, variant descriptions and pathogenicity predictions of these sequence variations are shown in Table 1.
Table 1. Clinical phenotypes of patient, variant descriptions and pathogenicity prediction of the variants identified in this study.
| S. No. | Case ID | Origin of patient | Diagnosis | Gene | OMIM | Disease-Causing Mutation | Exon/ Intron | ExAC AltFreq_All | Zygosity | SIFT | PolyPhen2 | MutationTaster2 | MAPP | Align GVGD Class | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27485 | Switzerland | adRP | RHO | 136880 | NM_000539.3:c.170T>G:p.Leu57Arg | Ex 1 | - | Heterozygous | Deleterious | Probably Damaging (0.991) | Disease causing | Bad | C0 | Sullivan (2006) Invest Ophthalmol Vis Sci 47: 3052 |
| 2 | 23880 | Switzerland | adRP | PRPF8 | 600059 | NM_006445.3:c.7000dup:p.Tyr2334Leufs*51 | Ex 43 | - | Heterozygous | NA | NA | NA | NA | NA | This study |
| 3 | 27536 | Turkey | arRP DD: LCA | RPGRIP1 | 613826 | NM_020366.3:c.2890del:p.Ser964Profs*37 | Ex 17 | - | Homozygous | NA | NA | NA | NA | NA | This study |
| 4 | 24718 | Switzerland | arCRD | BEST1 | 611809 | NM_001139443.1:c.242G>A:p.Arg81His | Ex 3 | 0.00012 | Homozygous | Deleterious | Probably Damaging (1.0) | Disease causing | Good | C0 | Krämer (2000) Eur J Hum Genet 8: 286 |
| 5 | 26165 | Switzerland | adRP | RP1 | 180100 | NM_006269.1:c.2613dup:p.R872Tfs*2 | Ex 4 | - | Heterozygous | NA | NA | NA | NA | NA | Payne (2000) Invest Ophthalmol Vis Sci 41: 4069 |
| 6 | 25900 | Switzerland | adRP | RP1 | 180100 | NM_006269.1:c.2613dup:p.R872Tfs*2 | Ex 4 | - | Heterozygous | NA | NA | NA | NA | NA | Payne (2000) Invest Ophthalmol Vis Sci 41: 4069 |
| 7 | 26007 | Switzerland | arRP | CDHR1 | 613660 | NM_033100.2:c.398C>G:p.Pro133Arg& NM_033100.2:c.439-17G>A | Ex 5 & Int 5 | - & 0.00002 | Compound heterozygous | Deleterious & NA | Probably Damaging (1.0) & NA | Disease causing & NA | Bad & NA | C0 & NA | This study |
| 8 | 23530 | Switzerland | adRP | PRPF31 | 600138 | NM_015629.3:c.548_580dup:p.Glu183_Met193dup | Ex 7 | - | Heterozygous | NA | NA | NA | NA | NA | This study |
| 9 | 22538 | Switzerland | arCRD | - | - | Not yet identified | - | - | - | - | - | - | - | - | - |
| 10 | 26309 | Switzerland | adRP | - | - | Not yet identified | - | - | - | - | - | - | - | - | - |
| 11 | 23609 | Switzerland | arRP | - | - | Not yet identified | - | - | - | - | - | - | - | - | - |
All identified variants described in this study (except RP1 and BEST1variants) were absent in an in-house database of 130 exomes (260 alleles). RP1 variant (NM_006269.1:c.2613dup) was identified in four additional families diagnosed with retinitis pigmentosa. BEST1 variant (NM_001139443.1:c.242G>A) was heterozygous in an individual not known to be affected by retinal disease (allele frequency = 0.38% in our in-house database).
WES analyses
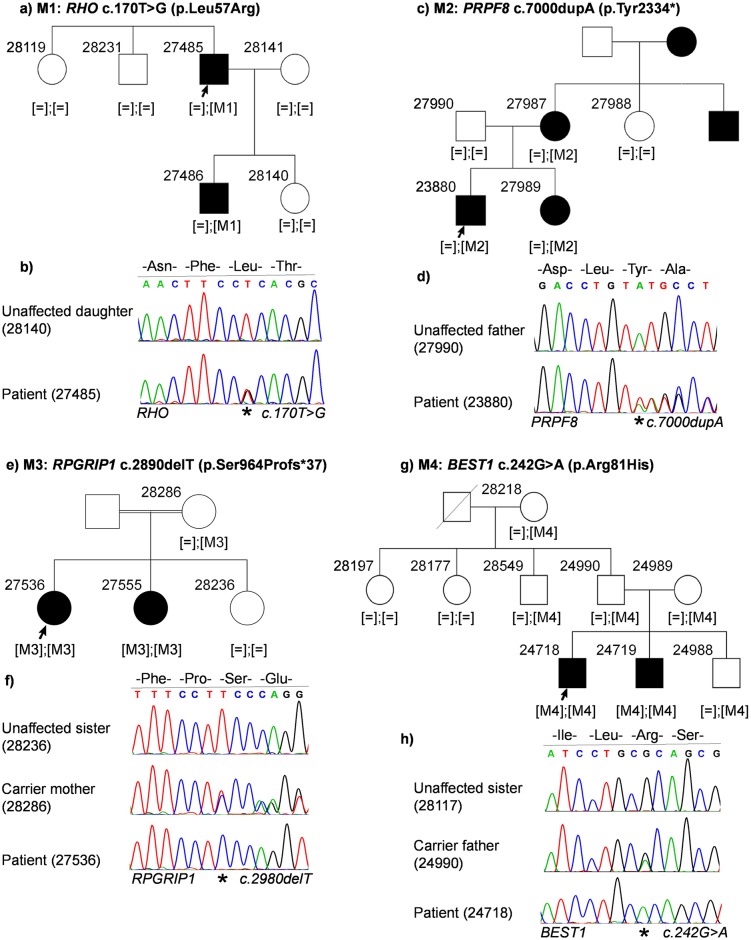
Case ID 27485 (RP, autosomal dominant): The index patient is a 63-year old male patient diagnosed with RP. The family presented an autosomal dominant mode of inheritance with the son of the index patient also being affected. We identified a heterozygous missense mutation in RHO: NM_000539.3:c.170T>G:p.Leu57Arg (Fig 1a and 1b). This mutation was previously described[19] (HGMD_PRO = CM063096) and cosegregated with the disease in the family. The mutation is predicted to be damaging by four protein prediction algorithms (Table 1) and is located in the transmembrane domain.
Fig 1. Patient pedigrees and sequence chromatography of identified disease-associated variants.
Affected individuals are indicated with filled symbols and unaffected individuals are indicated with open symbols. Index patients are indicated with an arrow and were subjected to WES. Variants are denoted as M1, M2, M3 and M4 and zygosity of variants are indicated in third brackets below every analyzed family member. Sequence chromatography images of patients and representative family members are shown below the reference sequence. Mutated position on the chromatograph is depicted with an asterix. (a) Family pedigree of patient 27485. (b) Sequence chromatography of identified heterozygous RHO variant in patient (bottom) and unaffected daughter (top). (c) Family pedigree of patient 23880. (d) Sequence chromatography of identified heterozygous PRPF8 variant in the patient (bottom) and unaffected father (top). (e) Family pedigree of patient 27536. (f) Sequence chromatography of identified homozygous RPGRIP1 variant in the patient (bottom), heterozygous unaffected mother (middle) and unaffected sister (top). (g) Family pedigree of patient 24718. (h) Sequence chromatography of identified homozygous BEST1 variant in patient (bottom), unaffected heterozygous father (middle) and unaffected sister (top).
Case ID 23880 (RP, autosomal dominant): The index patient is a 35-year old male with an affected sibling. His mother was also affected by RP but not his father. There were additional affected members in the maternal branch of the family, clearly pointing towards an autosomal dominant inheritance pattern. A novel heterozygous frameshift duplication was identified in PRPF8 in the penultimate codon: NM_006445.3:c.7000dupA:p.Tyr2334* (Fig 1c and 1d). This variant was identified in all affected family members (patient, his sister and father). Unaffected family members did not carry this variant.
Case ID 27536 (RP, autosomal recessive; differential diagnosis: Leber congenital amaurosis, LCA): The patient is a 32-year old female of consanguineous parents who are not affected. She has one affected and one unaffected sister. A novel homozygous frameshift deletion was identified in exon 17 (out of 24 exons) of RPGRIP1 in the patient and the affected sister: NM_020366.3:c.2890delT:p.Ser964Profs*37 (Fig 1e and 1f). This mutation leads to a frameshift and a premature stop codon 37 triplets downstream from the mutation. NGS variant calling initially suggested a heterozygous mutation in RPGRIP1. The mutation was excluded in the unaffected sister and the mother. Samples from the father of the patient were not available, but it can be assumed that, like the mother, he also is a carrier for this mutation.
Case ID 24718 (Cone-rod dystrophy, autosomal recessive): A homozygous missense mutation in BEST1 was identified in the affected index patient (24718) and his affected brother (24719): NM_001139443.1:c.242G>A:p.Arg81His. They inherited one mutant allele from each parent; both heterozygous carriers for the mutation (Fig 1g and 1h). All other family members are unaffected and do not show any bestrophin-associated manifestations. They are either heterozygous carriers (28218, 28549, 24990 and 24989) or non-carriers (28197 and 28177) of the mutation. This mutation has previously been reported as disease-causing[20–22] (HGMD_PRO = CM001382). It is predicted to be deleterious (SIFT), probably damaging (Polyphen2 score = 1.0) and disease-causing (MutationTaster2).
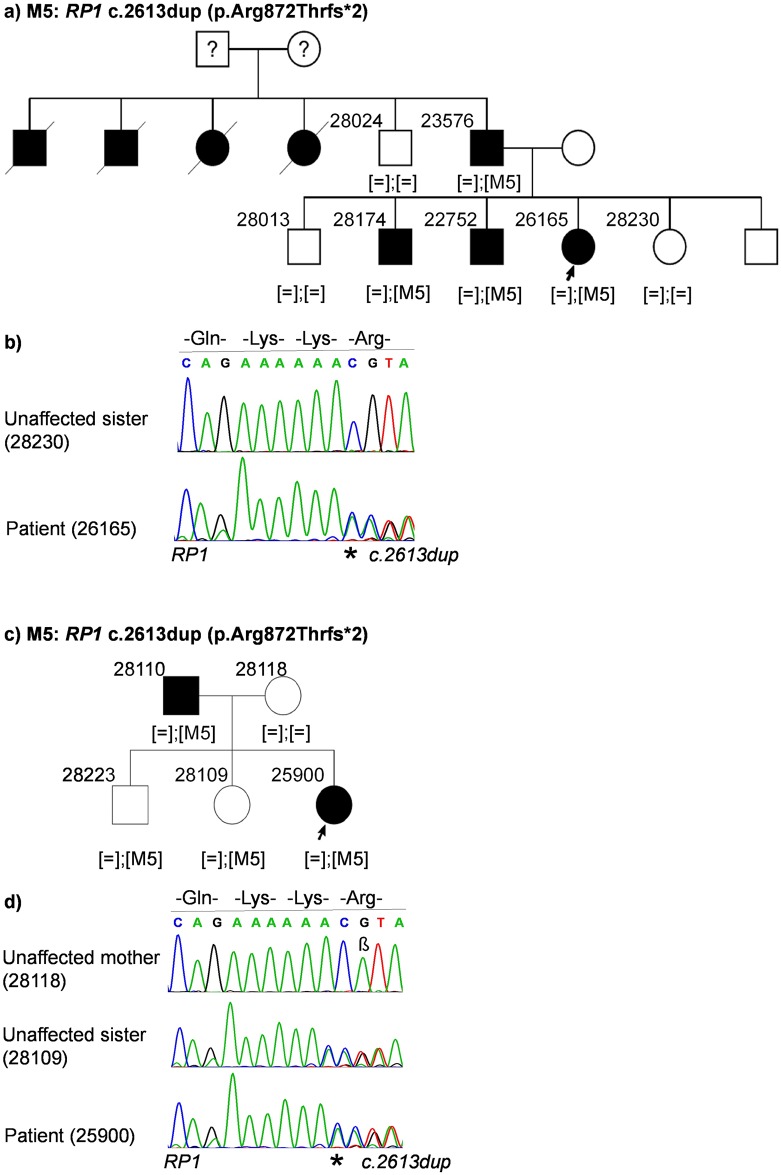
Case ID 26165 (RP, autosomal dominant): The index patient is a 54-year old female with two affected brothers, 3 unaffected siblings, an affected father and unaffected mother. The pedigree indicates an autosomal dominant inheritance pattern (Fig 2a). A heterozygous frameshift insertion was found in exon 4 in RP1 (last exon): NM_006269.1:c.2613dup:p.Arg872Thrfs*2 (Fig 2b). This frameshift leads to a premature stop codon, 2 triplets downstream from the mutation. The mutation co-segregated with the disease within the family (Fig 2a). It has previously been described as disease-associated[23] (HGMD_PRO = CI004598). The mutation was absent in an ethnically matched control cohort representing 576 autosomal alleles.
Fig 2. Patient pedigrees and sequence chromatography of identified disease-associated variants.
Variants are denoted as M5. (a) Family pedigree of patient 26165. (b) Sequence chromatography of identified heterozygous RP1 variant in patient (bottom) and unaffected sister (top). (c) Family pedigree of patient 25900. (d) Sequence chromatography of identified heterozygous RP1 variant in patient (bottom), unaffected sister carrying the variant (middle) and unaffected mother (top). β: A known polymorphism at position c.2618 in RP1 gene with a frequency of 26.98% in Europeans (Source: Exome Aggregation Consortium).
Case ID 25900 (RP, autosomal dominant): The index patient is a 47-year old female with an affected father. Her mother and two siblings are unaffected (Fig 2c). The same frameshift insertion in RP1 as described above was found in the patient and the affected father: NM_006269.1:c.2613dup:p.Arg872Thrfs*2. The mutation was also identified in two additional members in this family (28223 and 28109) who did not present any symptoms of the disease at the first clinical examination at the age of 37 and 40 years, respectively (Fig 2c and 2d).
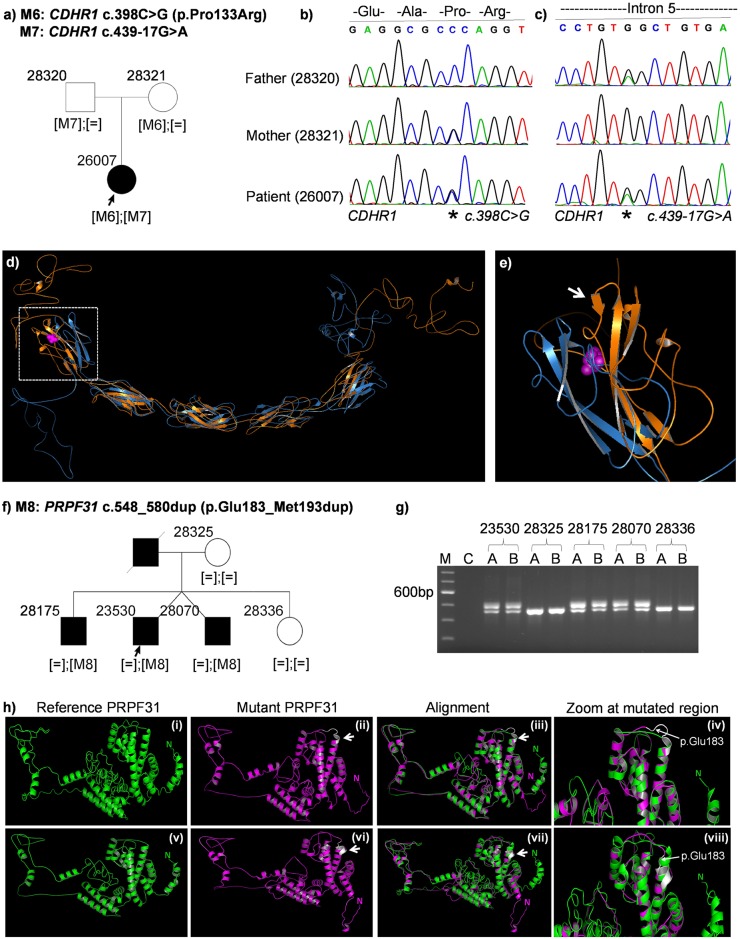
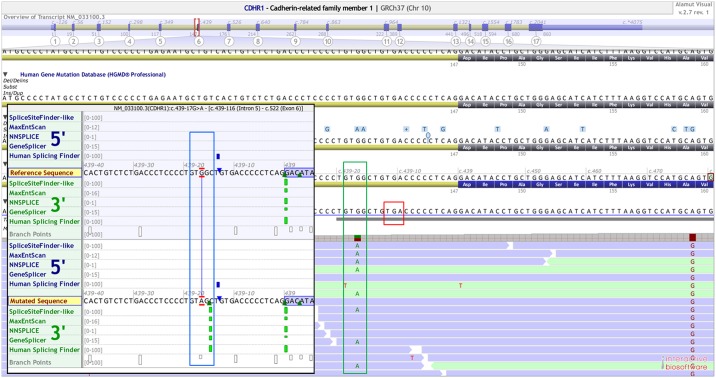
Case ID 26007 (RP, autosomal recessive): The patient is a 59-year old woman clinically diagnosed with RP. Her parents were not affected (Fig 3a). We found novel, compound heterozygous mutations in CDHR1, encoding the cadherin-related family member 1, in the DNA of the patient. She inherited one mutant allele from each parent (Fig 3b and 3c). (i) NM_033100.2:c.398C>G:p.Pro133Arg (Missense) was maternally transmitted. It leads to the change of a highly conserved Proline to Arginine (S1 Fig, red rectangle). It is predicted to be deleterious (SIFT) and disease-causing (MutationTaster2). Structural prediction using iTasser showed that this missense variant leads to the creation of a short beta-sheet in the mutant protein which lies in the first cadherin domain of CDHR1 (Fig 3d and 3e). (ii) NM_033100.2:c.439-17G>A was inherited from her father. This variant is located 17 bases upstream of exon 6 and predicted to generate a cryptic splice acceptor site in intron 5 (Fig 4, green rectangle). The prediction values of the new splice acceptor using five different splice prediction algorithms (SpliceSite Finder-like, MaxEntScan, NNSPLICE, GeneSplicer and Human Splicing Finder) are comparable to that of the canonical splice acceptor site beginning at the conserved splice acceptor of exon 6 (Fig 4 inset: blue rectangle, Table 2). It seems likely that this new splice acceptor site is used by the splicing machinery in addition to the canonical site. This leads to a 15bp longer exon and generate a stop codon 3-basepairs downstream of the cryptic splice acceptor site (Fig 4, red rectangle). Both variants were absent in an ethnically matched control cohort of 576 alleles.
Fig 3. Patient pedigrees and sequence chromatography of identified disease-associated variants.
Variants are denoted as M6, M7 and M8. (a) Family pedigree of patient 26007. (b) Sequence chromatography of heterozygous CDHR1 variant c.398C>G in patient (bottom), carrier mother (middle) and father (top). (c) Sequence chromatography of heterozygous CDHR1 variant c.439-17G>A in patient (bottom), mother (middle) and carrier father (top). (d) Predicted structure of CDHR1 reference protein (in blue) aligned to mutant CDHR1 (p.Pro133Arg) (in orange). The mutation is shown by magenta spheres and is localized within the first cadherin domain (white rectangle). (e) A zoomed image of the first cadherin domain of CDHR1 shows an additional beta-sheet (white arrow) close to the mutation (f) Family pedigree of patient 23530. (g) Agarose gel image of PRPF31 exon 7 PCR shows a larger band only in affected members indicating a duplication. C = Water control in PCR. (h) Comparison of predicted models of the PRPF31 reference protein sequence (i & v, in green), mutant PRPF31 (ii & vi, in magenta), alignment of reference and mutant PRPF31(iii & viii) and zoomed image of the alignment at the mutation site (iv & viii). An additional turn of the mutant in the coiled-coil domain is depicted in white. The first amino acid of the 11bp duplication is shown by a white arrow. “N” denotes the N-terminus of the protein.
Fig 4. CDHR1 intronic variant.
Snapshot of Alamut visual showing an intronic variant (c.439-17G>A) in patient 26007 (green rectangle). The bam alignment file clearly shows a heterozygous variant 17bp upstream from exon 6. Inset: Snapshot of splicing prediction algorithms (in silico) shows a strong cryptic splice acceptor gain at the site of the variant (blue rectangle). Values are comparable to that of canonical splice acceptor site. Red rectangle shows a stop codon in-frame to the cryptic splice activator site.
Table 2. Prediction scores of canonical (c.439) and cryptic splice site (c.439-17) by five prediction algorithms due to the variant c.439-17G>A in CDHR1 gene.
| Canonical acceptor site (c.439) | Cryptic acceptor site (c.439-17) | ||
|---|---|---|---|
| SpliceSiteFinder-like | 80.5 | Not predicted | |
| MaxEntScan | 8.2 | Not predicted | |
| Reference | NNSPLICE | 0.7 | Not predicted |
| GeneSplicer | 5.7 | Not predicted | |
| Human Splicing Finder | 89.6 | Not predicted | |
| SpliceSiteFinder-like | 80.5 | 71.8 | |
| MaxEntScan | 6.5 | 9.2 | |
| Mutant | NNSPLICE | 0.8 | 0.7 |
| GeneSplicer | 3.8 | 8.1 | |
| Human Splicing Finder | 89.6 | 83.2 |
Since the splice site is followed by a stop codon, we reasoned that the mutant transcript might be affected by nonsense-mediated mRNA decay. To test this hypothesis, we treated fibroblasts established from a skin biopsy of the patient in comparison to control fibroblasts (not carrying these mutations in CDHR1) with cycloheximide and DMSO as control. Subsequently, we isolated total RNA from these cell lines and performed RT-PCR. Upon gel electrophoresis, we identified an aberrant RT-PCR product (Fig 5b, white asterix) in the patient cell line treated with cycloheximide. The expected size of this product was 140bp. No product was amplified in the control cell line after DMSO or cycloheximide treatment, and in the patient cell line treated with DMSO (Fig 5b). Upon sequencing this PCR product, we confirmed that the predicted splice site activation occurred in this patient. It included a 15bp insert from intron 5 of CDHR1 which corresponds to the predicted cryptic splice activation occurring due to the mutation c.439-17G>A (Figs 5c and 4 inset).
Fig 5. Analysis of alternative splicing in vitro.
(a) Primer design to capture putative intron retention due to c.439-17G>A variant in CDHR1. (b) Agarose gel electrophoresis of RT-PCR showing a 140bp PCR product only in the patient cell-line (white asterix). No products were seen in control cell line or water control. (c) Bottom: Sequence chromatograph shows a clear retention of 15bp from intron 5 in the RT-PCR product, generated due to the cryptic splice-site activation. Top: Schematic representing the exon-intron boundaries as observed in the RT-PCR product.
Case ID 23530 (RP, autosomal dominant): The index patient is a 43-year old male with an affected twin brother, a second affected brother, an unaffected sister and unaffected mother (Fig 3f). The father was reported to be affected. Among the known RP genes, no mutation explaining the disease was identified by using WES in this patient. However, upon screening of candidate genes by Sanger sequencing, a novel 33 base-pair duplication was found in the PRPF31 gene (NM_015629.3:c.548_580dup: p.Glu183_Met193dup), which cosegregated with the disease in the family. The duplication was observed only in affected family members as seen by a larger PCR product on the agarose gel (Fig 3g). Structural analysis of the PRPF31 mutant protein aligned to the reference protein structure predicts the generation of an additional turn due to this 11 amino acid duplication in the second coiled-coil alpha helix domain of the PRPF31 protein (Fig 3h). The model with the highest confidence score (c-score) predicted that the additional turn was in continuation with the existing alpha helix (Fig 3hi–3hiv). A second model suggested that this additional helix turn looped out of the coiled coil domain (Fig 3hv–3hviii). In both models, the mutation is predicted to lead to loss of the N-terminal alpha-helix (Fig 3h, denoted by the letter N in green for the reference PRPF31 and magenta for the mutant PRPF31).
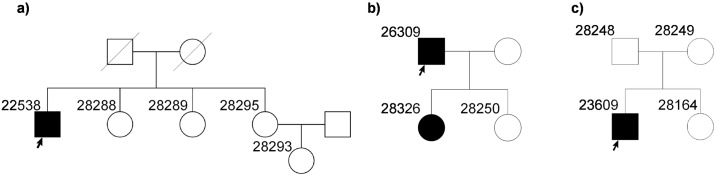
In three families (one with a putative dominant mode of inheritance and two potentially recessive cases), we were not able to detect mutations in genes currently associated with IRDs, which could explain the disease phenotype based on the implicated mode of inheritance (Fig 6). These cases are being further investigated for identification of novel disease associated gene mutations.
Fig 6. Pedigrees of families where underlying genetic mutations were not identified.
(a) Family pedigree of patient 22538 diagnosed with CORD. (b) Family pedigree of patient 26309 diagnosed with RP. (c) Family pedigree of patient 23609 diagnosed with CORD.
Discussion
IRDs show an extraordinary genetic and phenotypic heterogeneity. These diseases of the retina are currently associated with mutations in over 250 genes. With the advent of next generation sequencing (NGS), identification of the underlying genetic basis of IRDs has been revolutionized. Genetic diagnostics by means of panel or whole exome sequencing has been shown to be a reliable and fast method with a reported diagnostic efficiency between 50–65%[15, 24–27] (Tiwari et al, unpublished data). Although this number is higher compared to conventional Sanger sequencing and candidate gene approaches, a considerable percentage of cases still remains undiagnosed. Those undiagnosed patients are less frequent for syndromic cases[15] and in cases within consanguineous populations, where autozygosity-guided mutation analysis in combination with WES/NGS was shown to reveal higher diagnostic efficiency[28, 29]. We employed the well-established method of patient sample screening for mutations in most likely candidate genes and confirmed the identified variants in additional family members by segregation analysis. Some of the patients were prescreened for mutations in likely candidate genes (RHO, PRPH2, RP2, RPGR, CRX, NRL) using Sanger sequencing. In this study, which included one consanguineous family, we were able to identify disease-causing variants in 7 out of 11 cases using WES. A disease-associated variant in an additional case was identified only by Sanger sequencing. Our cohort consisted of 8 RP cases and 3 CORD cases, seven and one of whom revealed positive findings in this study, respectively. We identified 3 previously described mutations in 4 families and 5 novel variants in 4 families. The variant types included 3 frameshift, 3 missense and 1 splice mutation as well as 1 duplication of 33bp.
Although it has led to a higher diagnostic success rate, carefulness is needed during NGS data analysis, especially in diseases with a remarkable genetic heterogeneity, such as IRDs. The identification of disease-associated variants sometimes is very straightforward as in case 27485 (Fig 1a), where a known missense mutation in RHO was identified in the affected family members. Mutations in RHO account for the majority of autosomal dominant RP cases. It can be slightly more difficult when very few mutations have been identified in a gene. For example in case 23880 (Fig 1c), in whom a novel, single nucleotide duplication in the penultimate codon of PRPF8 was identified. Mutations in PRPF8 are one of the least frequent causes of dominant RP and therefore this gene is less likely to be screened by Sanger sequencing in traditional diagnostic laboratory screening pipeline. Amino acids 2301–2335 at the C-terminus of PRPF8 are known to interact with EFTUD2 and SNRNP200[30–32] and many mutations leading to RP have been shown to cluster at the C-terminal domain of PRPF8[30, 33]. An inefficient repression of SNRNP200 due to mutations in the C-terminus of PRPF8 has been hypothesized to be associated with PRPF8-linked RP[34]. The nonsense mutation p.Tyr2334* in PRPF8 in patient 23880 generates a premature termination codon in the protein. However, being in the penultimate amino acid, loss of function cannot be explained by nonsense mediated decay. Most likely, it leads to an inefficient repression of SNRNP200 function or loss of interaction with either EFTUD2 or SNRNP200.
If a panel approach is being used, the analysis is restricted to genes associated with a specific disease. For example in case 27536 (Fig 1e), the primary clinical diagnosis was RP with a differential clinical diagnosis of Leber congenital amaurosis (LCA). A RP panel will typically exclude RPGRIP1, because majority of the mutations described in RPGRIP1 have been associated with LCA (n = 77, source: HGMD), while very few with RP (n = 4, source: HGMD), thus leading to an unsuccessful genetic diagnosis. In this case we identified a novel frameshift deletion in all affected family members in exon 17 (out of 24) of RPGRIP1 (p.Ser964Profs*37), which leads to a premature termination codon 37 triplets downstream from the mutation and most likely results in non-sense mediated decay of the transcript. Retrospectively, the differential diagnosis of LCA could more aptly describe the clinical phenotype in this patient. Similarly in case 24718, CORD is due to a previously described homozygous missense mutation in BEST1 (p.Arg81His)[20]. Although the majority of BEST1 mutations are dominantly inherited, mutations in BEST1 are associated with both recessive and dominant forms of Best macular dystrophy[35]. BEST1 is not the most likely candidate for CORD, and as such, BEST1 would be excluded from a typical CORD diagnostic gene panel. Functional studies have shown that this mutation leads to reduced chloride conductance and increased proteasomal degradation of the BEST1 protein [21, 22, 36].
Analysis pipelines and variant-calling algorithms are being improved continuously and therefore chances of a wrong call can pose problems with analysis. For example in case 27536, zygosity of the RPGRIP1 mutation c.2890delT was annotated to be heterozygous instead of homozygous. Since, the parents were consanguineous and unaffected, we expected in this case a homozygous or compound heterozygous variant(s) to be causative for the disease. Only upon a detailed reanalysis of this specific mutation, we identified that the sequence included 6 T-allele reads and 49 deletions of the T-allele, suggesting atypical allele balance (<20% reference allele) in combination with a strand bias of the T-allele (S2 Fig). Upon verification by Sanger, we could confirm this deletion to be homozygous.
Gene mutations, which could be either recessively or dominantly inherited, or show incomplete penetrance, are particularly challenging in making precise interpretation of the genetic data and results, an example being RP1. In families of patients 26165 and 25900, a previously described dominantly inherited frameshift duplication in RP1 was identified[23] (Fig 2a–2d). In the family of patient 26165, all affected members were heterozygous for the mutation and non-affected family members did not carry the mutation. However, in the family of patient 25900, two unaffected siblings (one brother and one sister) were also heterozygous for the mutation (Fig 2c and 2d). This could be due to incomplete penetrance as dominant mutations in RP1 have been shown to exhibit variable expressivity[37, 38]. A later age of onset of the disease in these family members could also explain this discrepancy. Therefore, a clinical re-examination would be required in these cases. We have also identified this mutation in four additional families with autosomal dominant RP in our cohort (data not shown), supporting the pathogenicity of the sequence variant.
CDHR1 belongs to the cadherin superfamily of calcium-dependent cell adhesion molecules. It encodes a photoreceptor-specific cadherin that plays a role in outer segment disc morphogenesis. It may be required for the structural integrity of the outer segment of photoreceptor cells and has been shown to interact with PROM1[39]. We identified compound heterozygous mutations in a trio where the index patient was affected with RP (Fig 3a). The missense variant in CDHR1 (Pro133Arg) affects a highly conserved amino acid and is localized in the first cadherin domain of the protein. Structural analysis predicted a new beta-sheet in this cadherin domain due to the missense variant. Cadherins are involved in calcium-dependent cell-cell adhesion. Another proline substitution has been identified at the end of the fifth cadherin domain in a Spanish RP patient[40]. It can be hypothesized that the mutation perturbs the cell-cell adhesion role of the cadherin domain. Alternatively it could mediate its pathogenicity by a mechanism not yet described for CDHR1. The variant c.439-17G>A of CDHR1 was predicted to activate a cryptic splice activator site in silico. Using fibroblasts from the patient, this activation was confirmed in a patient-derived cell line. Often, the focus of mutation identification lies within the coding regions. Splice changes affecting positions +/-2 and +5 base-pairs around exons are also given importance. However, padding regions could also harbor mutations which could lead to alternative splicing. These regions vary greatly between exons and depend upon the enrichment method being used. In addition, variants lying at the extremities of padding regions typically show strand bias and thus get excluded from many analysis pipelines. It is therefore important to carefully analyze the “padding” region of the exome capturing sequences, in order to not miss mutations lying further away from the canonical splice acceptor and donor sites. This also highlights the necessity of functional assays to validate predicted effects of variants e.g., missense or splice-site changes. In this case, only the transcript analysis of the patient-derived cell line confirmed the splice defect.
In patient 23530, none of the variants identified upon WES explain the dominant RP phenotype in the family. We included the patients’ DNA in a Sanger screening project of PRPF31 gene, mutations in which account for the second highest number of disease-associated mutations in autosomal dominant RP cases. With Sanger sequencing, a novel 33bp duplication was identified in PRPF31 in the patient and all affected family members. This case illustrates an important limitation of this NGS approach, where a large duplication was missed in WES data but could only be identified by conventional Sanger sequencing. Structural analysis predicts the generation of an additional turn in the alpha helix of the coiled-coil domain of the protein. It is likely that the mutation perturbs the interaction of PRPF31 with PRPF6[41]. Improved bioinformatics algorithms to detect duplications or deletions larger than 20 base pairs might help in identifying such mutations. However, such specific analyses are not routinely used and therefore it is important to consider using alternative analysis methods for cases still lacking a genetic diagnosis.
In conclusion, our study shows the power of WES in identifying pathogenic mutations in IRDs with a success rate of 63% but also its limitations. There might be multiple mutations per case in different genes, in addition to de-novo sequence variations that might not co-segregate with the disease[15]. Moreover, mutations located within and outside of the captured exonic regions should be carefully evaluated for effects on splicing or additional regulatory effects. In addition, a complementary approach to WES can be very helpful to identify IRD-associated mutations in cases lacking a genetic diagnosis.
Supporting Information
This variant affects an amino acid that is conserved from Tetraodon to humans (red rectangle).
(TIF)
This 1bp deletion leading to frameshift was annotated as heterozygous. Values of the reads in inset show 49 deletions and 6 T-alleles (which shows a strand bias). Sanger sequencing confirmed this deletion to be homozygous.
(TIF)
Acknowledgments
The authors thank all patients and their family members for participating.
Data Availability
All scientific data that helps in understanding of the paper such as Sanger sequencing, pedigree of the family involved, sequencing and variant filtering pipelines are presented within the paper and its Supporting Information files. Abiding by patient privacy laws, the personal information of the patient and family members such as name, address, date of birth, patient ID, etc. will not be made public. This information does not in any way add or take away from the scientific content of the paper or hinder in its complete understanding.
Funding Statement
This study was funded by a grant from the Swiss National Science Foundation (Grant Number: 320030_138507) to WB and JN and from Velux Stiftung, Switzerland to WB. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Berger W, Kloeckener-Gruissem B, Neidhardt J. The molecular basis of human retinal and vitreoretinal diseases. Progress in retinal and eye research. 2010;29(5):335–75. 10.1016/j.preteyeres.2010.03.004 . [DOI] [PubMed] [Google Scholar]
- 2.Ellingford JM, Barton S, Bhaskar S, O'Sullivan J, Williams SG, Lamb JA, et al. Molecular findings from 537 individuals with inherited retinal disease. Journal of medical genetics. 2016. 10.1136/jmedgenet-2016-103837 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corton M, Nishiguchi KM, Avila-Fernandez A, Nikopoulos K, Riveiro-Alvarez R, Tatu SD, et al. Exome sequencing of index patients with retinal dystrophies as a tool for molecular diagnosis. PloS one. 2013;8(6):e65574 10.1371/journal.pone.0065574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang L, Xiao X, Li S, Jia X, Wang P, Sun W, et al. Molecular genetics of cone-rod dystrophy in Chinese patients: New data from 61 probands and mutation overview of 163 probands. Experimental eye research. 2016;146:252–8. 10.1016/j.exer.2016.03.015 . [DOI] [PubMed] [Google Scholar]
- 5.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–60. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25(16):2078–9. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic acids research. 2010;38(16):e164 10.1093/nar/gkq603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nature protocols. 2009;4(7):1073–81. 10.1038/nprot.2009.86 . [DOI] [PubMed] [Google Scholar]
- 9.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nature methods. 2010;7(4):248–9. 10.1038/nmeth0410-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nature methods. 2014;11(4):361–2. 10.1038/nmeth.2890 . [DOI] [PubMed] [Google Scholar]
- 11.Stone EA, Sidow A. Physicochemical constraint violation by missense substitutions mediates impairment of protein function and disease severity. Genome research. 2005;15(7):978–86. 10.1101/gr.3804205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathe E, Olivier M, Kato S, Ishioka C, Hainaut P, Tavtigian SV. Computational approaches for predicting the biological effect of p53 missense mutations: a comparison of three sequence analysis based methods. Nucleic acids research. 2006;34(5):1317–25. 10.1093/nar/gkj518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tavtigian SV, Deffenbaugh AM, Yin L, Judkins T, Scholl T, Samollow PB, et al. Comprehensive statistical study of 452 BRCA1 missense substitutions with classification of eight recurrent substitutions as neutral. Journal of medical genetics. 2006;43(4):295–305. 10.1136/jmg.2005.033878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, et al. Primer3—new capabilities and interfaces. Nucleic acids research. 2012;40(15):e115 10.1093/nar/gks596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glockle N, Kohl S, Mohr J, Scheurenbrand T, Sprecher A, Weisschuh N, et al. Panel-based next generation sequencing as a reliable and efficient technique to detect mutations in unselected patients with retinal dystrophies. European journal of human genetics: EJHG. 2014;22(1):99–104. 10.1038/ejhg.2013.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glaus E, Schmid F, Da Costa R, Berger W, Neidhardt J. Gene therapeutic approach using mutation-adapted U1 snRNA to correct a RPGR splice defect in patient-derived cells. Molecular therapy: the journal of the American Society of Gene Therapy. 2011;19(5):936–41. 10.1038/mt.2011.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villegas J, McPhaul M. Establishment and culture of human skin fibroblasts. Current protocols in molecular biology / edited by Frederick M Ausubel [et al]. 2005;Chapter 28:Unit 28 3 10.1002/0471142727.mb2803s71 . [DOI] [PubMed] [Google Scholar]
- 18.Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nature protocols. 2010;5(4):725–38. 10.1038/nprot.2010.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan LS, Bowne SJ, Birch DG, Hughbanks-Wheaton D, Heckenlively JR, Lewis RA, et al. Prevalence of disease-causing mutations in families with autosomal dominant retinitis pigmentosa: a screen of known genes in 200 families. Investigative ophthalmology & visual science. 2006;47(7):3052–64. 10.1167/iovs.05-1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer F, White K, Pauleikhoff D, Gehrig A, Passmore L, Rivera A, et al. Mutations in the VMD2 gene are associated with juvenile-onset vitelliform macular dystrophy (Best disease) and adult vitelliform macular dystrophy but not age-related macular degeneration. European journal of human genetics: EJHG. 2000;8(4):286–92. 10.1038/sj.ejhg.5200447 . [DOI] [PubMed] [Google Scholar]
- 21.Davidson AE, Millar ID, Burgess-Mullan R, Maher GJ, Urquhart JE, Brown PD, et al. Functional characterization of bestrophin-1 missense mutations associated with autosomal recessive bestrophinopathy. Investigative ophthalmology & visual science. 2011;52(6):3730–6. 10.1167/iovs.10-6707 . [DOI] [PubMed] [Google Scholar]
- 22.Johnson AA, Bachman LA, Gilles BJ, Cross SD, Stelzig KE, Resch ZT, et al. Autosomal Recessive Bestrophinopathy Is Not Associated With the Loss of Bestrophin-1 Anion Channel Function in a Patient With a Novel BEST1 Mutation. Investigative ophthalmology & visual science. 2015;56(8):4619–30. 10.1167/iovs.15-16910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Payne A, Vithana E, Khaliq S, Hameed A, Deller J, Abu-Safieh L, et al. RP1 protein truncating mutations predominate at the RP1 adRP locus. Investigative ophthalmology & visual science. 2000;41(13):4069–73. . [PubMed] [Google Scholar]
- 24.Consugar MB, Navarro-Gomez D, Place EM, Bujakowska KM, Sousa ME, Fonseca-Kelly ZD, et al. Panel-based genetic diagnostic testing for inherited eye diseases is highly accurate and reproducible, and more sensitive for variant detection, than exome sequencing. Genetics in medicine: official journal of the American College of Medical Genetics. 2015;17(4):253–61. 10.1038/gim.2014.172 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao L, Wang F, Wang H, Li Y, Alexander S, Wang K, et al. Next-generation sequencing-based molecular diagnosis of 82 retinitis pigmentosa probands from Northern Ireland. Human genetics. 2015;134(2):217–30. 10.1007/s00439-014-1512-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beryozkin A, Shevah E, Kimchi A, Mizrahi-Meissonnier L, Khateb S, Ratnapriya R, et al. Whole Exome Sequencing Reveals Mutations in Known Retinal Disease Genes in 33 out of 68 Israeli Families with Inherited Retinopathies. Scientific reports. 2015;5:13187 10.1038/srep13187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y, Guan L, Shen T, Zhang J, Xiao X, Jiang H, et al. Mutations of 60 known causative genes in 157 families with retinitis pigmentosa based on exome sequencing. Human genetics. 2014;133(10):1255–71. 10.1007/s00439-014-1460-2 . [DOI] [PubMed] [Google Scholar]
- 28.Abu-Safieh L, Alrashed M, Anazi S, Alkuraya H, Khan AO, Al-Owain M, et al. Autozygome-guided exome sequencing in retinal dystrophy patients reveals pathogenetic mutations and novel candidate disease genes. Genome research. 2013;23(2):236–47. 10.1101/gr.144105.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aldahmesh MA, Safieh LA, Alkuraya H, Al-Rajhi A, Shamseldin H, Hashem M, et al. Molecular characterization of retinitis pigmentosa in Saudi Arabia. Molecular vision. 2009;15:2464–9. [PMC free article] [PubMed] [Google Scholar]
- 30.Pena V, Liu S, Bujnicki JM, Luhrmann R, Wahl MC. Structure of a multipartite protein-protein interaction domain in splicing factor prp8 and its link to retinitis pigmentosa. Molecular cell. 2007;25(4):615–24. 10.1016/j.molcel.2007.01.023 . [DOI] [PubMed] [Google Scholar]
- 31.Maeder C, Kutach AK, Guthrie C. ATP-dependent unwinding of U4/U6 snRNAs by the Brr2 helicase requires the C terminus of Prp8. Nature structural & molecular biology. 2009;16(1):42–8. 10.1038/nsmb.1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mozaffari-Jovin S, Santos KF, Hsiao HH, Will CL, Urlaub H, Wahl MC, et al. The Prp8 RNase H-like domain inhibits Brr2-mediated U4/U6 snRNA unwinding by blocking Brr2 loading onto the U4 snRNA. Genes & development. 2012;26(21):2422–34. 10.1101/gad.200949.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Shen J, Guarnieri MT, Heroux A, Yang K, Zhao R. Crystal structure of the C-terminal domain of splicing factor Prp8 carrying retinitis pigmentosa mutants. Protein science: a publication of the Protein Society. 2007;16(6):1024–31. 10.1110/ps.072872007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mozaffari-Jovin S, Wandersleben T, Santos KF, Will CL, Luhrmann R, Wahl MC. Inhibition of RNA helicase Brr2 by the C-terminal tail of the spliceosomal protein Prp8. Science. 2013;341(6141):80–4. 10.1126/science.1237515 . [DOI] [PubMed] [Google Scholar]
- 35.Boon CJ, Klevering BJ, Leroy BP, Hoyng CB, Keunen JE, den Hollander AI. The spectrum of ocular phenotypes caused by mutations in the BEST1 gene. Progress in retinal and eye research. 2009;28(3):187–205. 10.1016/j.preteyeres.2009.04.002 . [DOI] [PubMed] [Google Scholar]
- 36.MacDonald IM, Gudiseva HV, Villanueva A, Greve M, Caruso R, Ayyagari R. Phenotype and genotype of patients with autosomal recessive bestrophinopathy. Ophthalmic Genet. 2012;33(3):123–9. 10.3109/13816810.2011.592172 . [DOI] [PubMed] [Google Scholar]
- 37.Dietrich K, Jacobi FK, Tippmann S, Schmid R, Zrenner E, Wissinger B, et al. A novel mutation of the RP1 gene (Lys778ter) associated with autosomal dominant retinitis pigmentosa. The British journal of ophthalmology. 2002;86(3):328–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Audo I, Mohand-Said S, Dhaenens CM, Germain A, Orhan E, Antonio A, et al. RP1 and autosomal dominant rod-cone dystrophy: novel mutations, a review of published variants, and genotype-phenotype correlation. Human mutation. 2012;33(1):73–80. 10.1002/humu.21640 . [DOI] [PubMed] [Google Scholar]
- 39.Yang Z, Chen Y, Lillo C, Chien J, Yu Z, Michaelides M, et al. Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. The Journal of clinical investigation. 2008;118(8):2908–16. 10.1172/JCI35891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikopoulos K, Avila-Fernandez A, Corton M, Lopez-Molina MI, Perez-Carro R, Bontadelli L, et al. Identification of two novel mutations in CDHR1 in consanguineous Spanish families with autosomal recessive retinal dystrophy. Scientific reports. 2015;5:13902 10.1038/srep13902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu S, Li P, Dybkov O, Nottrott S, Hartmuth K, Luhrmann R, et al. Binding of the human Prp31 Nop domain to a composite RNA-protein platform in U4 snRNP. Science. 2007;316(5821):115–20. 10.1126/science.1137924 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This variant affects an amino acid that is conserved from Tetraodon to humans (red rectangle).
(TIF)
This 1bp deletion leading to frameshift was annotated as heterozygous. Values of the reads in inset show 49 deletions and 6 T-alleles (which shows a strand bias). Sanger sequencing confirmed this deletion to be homozygous.
(TIF)
Data Availability Statement
All scientific data that helps in understanding of the paper such as Sanger sequencing, pedigree of the family involved, sequencing and variant filtering pipelines are presented within the paper and its Supporting Information files. Abiding by patient privacy laws, the personal information of the patient and family members such as name, address, date of birth, patient ID, etc. will not be made public. This information does not in any way add or take away from the scientific content of the paper or hinder in its complete understanding.