Abstract
Background
Transitional cell carcinoma (TCC) of urinary bladder cancer is the most common malignancy in the urinary system. Genetic instability is an essential property of malignant neoplasms and could be evaluated by microsatellite analysis. Alterations in numerous microsatellite loci are already described in urinary bladder TCC. The aim of this study was to investigate the usefulness of only two microsatellite loci for the detection of bladder TCC, and their correlation with the major clinicopathological parameters.
Methods
We analyzed the tissue samples derived from 70 patients with histopathologically confirmed TCC of the urinary bladder, collected by transurethral resection, and samples of normal bladder mucosa derived from 40 patients with nonmalignant diseases. Microsatellite alleles GSN and D18S51 were amplified in paired samples of tissue and leukocyte DNA from each patient, and were analyzed by electrophoresis.
Results
Microsatellite alterations at either GSN or D18S51locus, or in both, were detected in 46 out of the 70 patients (65.71 %) with TCC, but not in the patients of the control group. We found a significant statistical correlation between the frequencies of patients with microsatellite alterations in the examined loci and all three grades of histopathological T-classification. No significant correlation was found regarding the stages or the occurrence of recidivism, metastasis or cancer-related death within the two-year follow-up period.
Conclusions
This study indicates that two selected microsatellite markers could have a potential value in clinical and pathological evaluation of urinary bladder TCC, especially regarding the prediction of tumor differentiation. Additional studies and further validation of the method are needed. Hippokratia 2015; 19 (3): 200-204.
Keywords: Urinary bladder cancer, microsatellite, instability, loss of heterozygosity
Introduction
Bladder cancer is the most common malignancy in the urinary system and is associated with high incidence and mortality in the industrialized countries, with significant impact on public health1-3. It has been estimated that smoking is the most frequent risk-factor, implicated in approximately half of all bladder cancer cases. Other well-established risk factors are occupational exposure to aromatic amines and polycyclic aromatic hydrocarbons, and genetic predisposition; while the links with dietary factors and environmental pollution are less evident4. Transitional cell carcinoma (TCC) is the most frequent histological type that accounts for nearly 90% of all bladder cancers5. At the time of diagnosis, the majority of patients present with superficial disease (restricted to the epithelium or the subepithelial connective tissue), while approximately 50% to 70% of patients develop disease recurrence with 10–40% of cases, ultimately progressing to muscle-invasive disease over five years6.
Genetic instability is one of the essential properties of malignant neoplasms7-11. At a molecular level, one of the most studied markers of genome instability are the alterations in selected microsatellite loci (alternatively termed short tandem repeats), Those repetitive sequences (1-5 base pairs in length) are clustered in small groups of 10-50 copies interspersed in the human genome and are highly polymorphic. Overall, two phenomena are described regarding the molecular analysis of those loci in clinical cancer research. Microsatellite instability (MSI) is observed as a length’s difference of the allele’s repetitive sequence in cancer tissue, when compared with the original length in the genomes of any nonmalignant cells, of the same patient. Secondly, loss-of-heterozygosity (LOH) occurs when one of the microsatellite alleles, present in constitutive (normal) DNA, is missing in the paired tumor sample DNA.
Thus far, numerous genetic alterations in different microsatellite loci are described in urinary bladder TCC8,12-14.
The aim of this study was to investigate the usefulness of two microsatellite loci for the detection of bladder TCC and their correlation with the major clinicopathological parameters.
Material and methods
Patients and samples
In this study, we analyzed tissue samples from 70 patients with histopathologically confirmed TCC of the urinary bladder, collected by transurethral resection of the bladder tumor (TURBT) at the University Clinic of Urology in Skopje, between October 2009 and March 2011. Sixty-one male and nine female patients with median age of 64.29 years (range 38-79) were included in the study. Clinicopathological parameters that were evaluated were: histopathological grade (G1, G2, and G3), stage (superficial: ≤pT1, and muscle-invasive: ≥pT2) according to WHO classification15, as well as the clinical history (regarding the occurrence of recidivism, metastasis and cancer-related death in the two-year follow-up period). Grading and tumor staging were recorded at the time when the tissue sample was obtained. We used as a negative control group, tissue samples of histologically normal urinary bladder mucosa, obtained by open retropubic prostatectomy from 34 male patients with benign prostate hyperplasia (BPH) or bladder biopsy during transurethral or open surgery from six female patients with nonmalignant disease. The patients that consisted the control group had similar median age and sex distribution with those from the TCC group. Venous blood (5 mL in a tube with 200 µl of 0.5 M EDTA) was collected from each patient.
DNA isolation
Genomic DNA was isolated from tissue and leukocyte samples from each patient by digestion with 200 µg/mL Proteinase K (Sigma-Aldrich, St. Louis, USA) at 55oC overnight, following by standard phenol-chloroform extraction and ethanol precipitation. Isolated DNA was dissolved in TE buffer (10 mM Tris; 1 mM EDTA, pH 8.0 at 25oC). The quantity and quality of isolated DNA were evaluated by UV-spectrophotometry at 260 nm and by agarose gel-electrophoresis, respectively. Figure 1.
Figure 1. Typical changes in allelic patterns representing the two types of microsatellite alterations. A. microsatellite instability (MSI) appears as shifted allelic bands in the tumour DNA. B. loss of heterozygosity (LOH). LOH is observed as a lack of one of the allelic bands in the tumour (T) (arrow) as compared to the heterozygous alleles from leukocytes (blood).
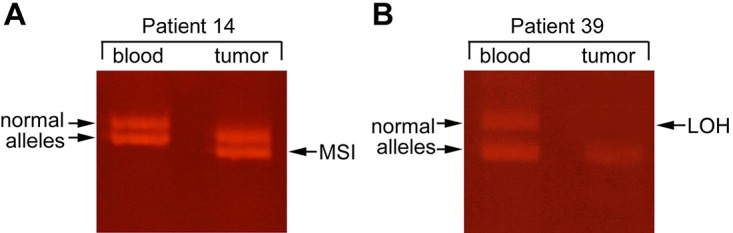
Microsatellite DNA analysis
Amplification of microsatellite loci GSN (dinucleotide repetition motif: 5’-CA-3’; located on chromosome 9) and D18S51 (tetranucleotide repetition motif: 5’-GAAA-3’; located on chromosome 18) was performed by polymerase chain reaction (PCR) as previously described by Berger et al13. PCR reagents were purchased from Sigma-Aldrich, St. Louis, USA and oligonucleotide primers were ordered from Sigma-Genosys, Cambridge, UK.
The amplified samples were resolved by denaturing polyacrylamide gel-electrophoresis (PAGE). Briefly, amplificates were diluted with equal volume of 95% formamide, 20 mM EDTA, 0.05% bromophenol blue, 0.05% xylene cyanol and analyzed by 6% PAGE in the presence of 7M urea (all reagents from Sigma-Aldrich, St. Louis, USA) in vertical electrophoresis cell Protean-II (Bio-Rad, Hercules, USA). Electrophoretic bands were visualized by fluorescent staining with ethidium bromide under UV-illumination (λmax=315 nm). Digital images were recorded and analyzed by ImageJ v.1.46r software (U. S. National Institutes of Health, Bethesda, Maryland, USA), freely available at: www.imagej.nih.gov/ij/download/, using gel electrophoresis analysis options. Microsatellite instability was considered when one of the electrophoretic bands (corresponding to amplified allele) had different mobility in comparison to the same band in the control leukocyte DNA of the same patient. Loss-of-heterozygosity was identified when the fluorescence intensity of one of the allele’s band was reduced by more than 40% compared to the intensity of the same band in the control leukocyte DNA of the same patient.
Statistical analysis
Differences between the observed and expected frequencies of normal or altered microsatellite alleles in patients’ samples and different clinicopathological data were analyzed by Chi-square with Yates’ continuity correction test and by Fisher’s exact probability (two-tailed) test using SPSS Statistics for Windows, v. 22.0 (IBM Corp. Armonk, USA). A p-value less than 0.05 was considered as statistically significant.
Ethics
The study was approved by the Ethical Committee of the Urology Clinic at the Medical Faculty in Skopje (No. 03-1165 from December 28, 2009) and signed informed consent was obtained from each patient recruited for the study.
Results
Microsatellite alterations (MSI and/or LOH) in either GSN or D18S51, or in both loci, were detected in 46 out of 70 patients (65.71%) with histopathologically confirmed urinary bladder TCC, but in none of the histologically normal bladder samples of the control group. The frequencies in patients with each type of microsatellite alterations are presented in Table 1.
Table 1. Frequency of microsatellite alterations in the DNA samples from 70 patients with urinary bladder TCC.
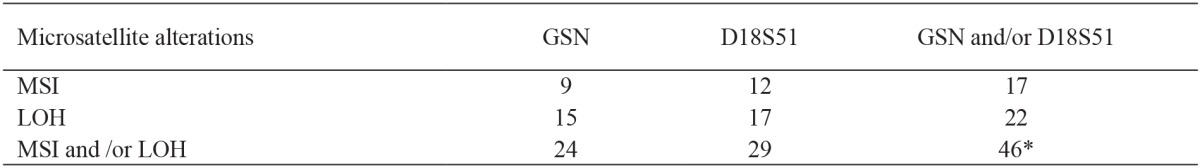
MSI: microsatellite instability, LOH: loss of heterozygosity, *: Total number of patients with any type of alteration (MSI and/or LOH) at either one or at both loci is lower than the sum of discrete numbers of MSI and LOH, since simultaneously alterations at both loci were observed in seven patients.
For the further analysis, the TCC patient group was stratified according to the main clinicopathological parameters. Given the relatively small observed frequencies, the occurrence of MSI and/or LOH in the two loci was considered as a microsatellite alteration in that patient for the purpose of statistical calculations (Table 2). Using the Chi-square test, we found statistically significant differences in the frequencies of patients with microsatellite alterations in the examined loci (either in GSN and D18S51), regarding the three grades of histopathological T-classification (p =0.044). This significance was additionally confirmed using Yates’ continuity correction test and Fisher’s exact test.
Table 2. Frequency of microsatellite alterations regarding the clinicopathological parameters.
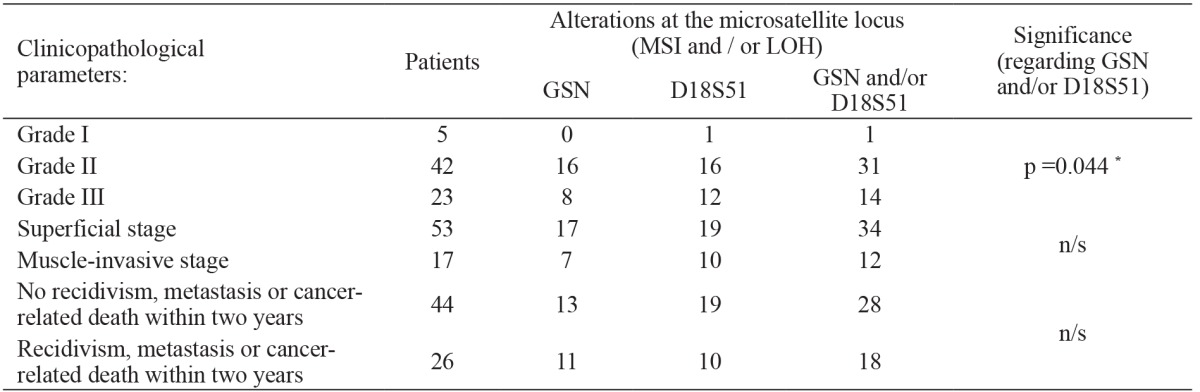
*: significant; n/s: non-significant.
On the contrary, the frequencies in patients with normal and altered microsatellite loci were not statistically significant when the correlation was calculated against the two histopathological stages: superficial (non-muscle-invasive) and muscle-invasive (p =0.847). Of the 70 analyzed patients, 44 (57.14%) have remained free of tumor recidivism, metastasis or cancer-related death within two years of obtaining the tissue sample. Twenty-six patients had either local tumor recidivism, distant metastasis or died from those causes during this evaluation period. The statistical analysis showed no significant correlation between frequencies of the microsatellite alterations in either or both of the examined loci in the two patients’ subgroups (p =0.634). According to further patients’ stratification, we analyzed the patients in which local recidivism occurred during the two-year follow-up period. In 53 patients initially diagnosed with non-muscle-invasive bladder TCC, local recurrence was recorded in 15 patients (28.3%), but we found no significant correlation between the frequencies of microsatellite alterations (p =0.772). No correlation was found regarding the preoperative urine cytology and presence of microsatellite alterations (data not shown).
Discussion
Initial and follow-up diagnosis of urinary bladder cancer is currently based on urine cytology, cystoscopy, histological analysis of tissue samples or biopsies, and imaging techniques. Limitations of those approaches, as well as the emerging discoveries regarding molecular processes involved in the cancer etiopathogenesis, has resulted in the development of new methods for prediction of tumor stage, grade, and potency for recurrence, as well as for cancer-related mortality. Numerous studies based on microsatellite alterations in bladder TCC have been published so far8,12,14. Investigating the panel of 17 microsatellite loci, Berger et al13 reported that the loci on chromosomes 9 and 18 proved to be the most informative. Since the microsatellite analysis is time-consuming, expensive, and requires highly-trained personnel, we investigated if using only two selected loci could have any potential practical value in the detection of urinary bladder cancer and also determined the specificity and the sensitivity of this molecular analysis.
In the present study, we examined matched tumor and blood DNA samples from patients with histopathologically confirmed bladder TCC, as well as from normal bladder mucosa from control group patients with nonmalignant diseases. Alterations in the two investigated microsatellite loci (GSN and/or D18S51) were found in 46 out of 70 patients with TCC, but none of the tissue samples from the control group. Thus, the calculated sensitivity of this analysis is 65.71%, and specificity is 100%. In the literature, the reported frequency of microsatellite alterations in urinary bladder TCC varies extremely from 0 to 100% detected12,16. Furthermore, considering the clinicopathological parameters, we found a significant correlation of microsatellite alterations only with the histopathological grades of tumor differentiation, but not with the pathological stages or with the occurrence of recidivism, metastasis, or cancer-related death during the two-year follow-up of the patients with TCC. Vaish et al17, using a different set of microsatellite loci, found a positive correlation with urinary bladder TCC grade, stage, and recurrence. This discrepancy could, at least in part, be due to the differences in the selection of microsatellite loci, their polymorphic nature and possible ethnic disparities in the patients’ populations.
The origin of those microsatellite alterations in cancer seems to be very distinctive. Mutational inactivation or transcriptional silencing of the DNA mismatch repair (MMR) genes (MLH1, MSH2, MSH6 or PMS2) results in an accumulation of DNA replicative errors, especially, within microsatellite repetition regions18. This can result in a so-called mutator phenotype, characterized by an increased frequency of mutations in tumor-suppressor genes and oncogenes19.
Also, higher frequencies of microsatellite instability were described in loci with tetranucleotide than in mono- and dinucleotide repeats in lung, head and neck, and bladder8, as well as in esophageal tumours20. Similarly, we found that the tetranucleotide repeats-locus D18S51 was more sensitive than dinucleotide repeat-locus GSN, although this difference has no statistical significance. However, Catto et al reported that the observed instability at selected microsatellite loci with tetranucleotide repeats in bladder cancers is not associated with reduced MMR expression, and that it could be due to an increased and prolonged exposure to some environmental carcinogenic agents14.
On the other hand, loss-of-heterozygosity can only be detected in loci that are heterozygous in that patient. Molecular mechanisms leading to LOH includes, but are not limited to, mitotic recombination, deletion, gene conversion, translocation, double-strand DNA breakage, chromosomal fusion or telomeric end-to-end fusions and loss of a large fragment or a whole chromosome21.
Interestingly, a recently published study indicated that histologically normal urothelium of patients with a history of TCC is genomically unstable22. Thus, it is realistic to expect that similar extent of microsatellite alterations could be expected to be identified in nearby epithelium around the malignant one. This could, at least in part, explain the high sensitivity of these markers even in tumor tissue samples obtained without microdissection.
Considering the relatively small number of patients, especially after stratification into distinct clinicopathological subgroups; a larger patient group is needed to confirm those data and the potential predictive value of this molecular analysis.
Conclusions
Microsatellite alterations at GSN and D18S51 loci were observed in almost two-third of the 70 patients with urinary bladder TCC. Statistically, significant correlation was found between the histopathological grades, but not with the stages or occurrence of local recidivism, metastasis and cancer-related death within the two-year follow-up period. However, given the relatively small size and heterogeneity of our patients’ groups, further studies with additional parameters and larger patient number, are needed to demonstrate the clinicopathological usefulness of microsatellite analysis of those two loci in the prediction of bladder urothelial cancer differentiation.
Conflict of interest
The authors have no financial disclosures or conflicts of interest.
Acknowledgment
Study was presented as an oral presentation at the SEEM2014, EAU 10th South Eastern European Meeting and its abstract is included both onilen and printed in the Eur Urol Suppl, 2014; 13(7) e1470.
References
- 1.Lokeshwar VB, Habuchi T, Grossman HB, Murphy WM, Hautmann SH, Hemstreet GP, 3rd, et al. Bladder tumor markers beyond cytology: International Consensus Panel on bladder tumor markers. Urology. 2005;66:35–63. doi: 10.1016/j.urology.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 2.Kakehi Y, Hirao Y, Kim WJ, Ozono S, Masumori N, Miyanaga N, et al. Bladder Cancer Working Group report. Jpn J Clin Oncol. 2010;40:i57–i64. doi: 10.1093/jjco/hyq128. [DOI] [PubMed] [Google Scholar]
- 3.Chu H, Wang M, Zhang Z. Bladder cancer epidemiology and genetic susceptibility. J Biomed Res. 2013;27:170–178. doi: 10.7555/JBR.27.20130026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Cordon-Cardo C. Molecular alterations associated with bladder cancer initiation and progression. Scand J Urol Nephrol Suppl. 2008:154–165. doi: 10.1080/03008880802291915. [DOI] [PubMed] [Google Scholar]
- 6.Nishiyama H, Habuchi T, Watanabe J, Teramukai S, Tada H, Ono Y, et al. Clinical outcome of a large-scale multi-institutional retrospective study for locally advanced bladder cancer: a survey including 1131 patients treated during 1990-2000 in Japan. Eur Urol. 2004;45:176–181. doi: 10.1016/j.eururo.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Cheng KC, Loeb LA. Genomic instability and tumor progression: mechanistic considerations. Adv Cancer Res. 1993;60:121–156. doi: 10.1016/s0065-230x(08)60824-6. [DOI] [PubMed] [Google Scholar]
- 8.Mao L, Lee DJ, Tockman MS, Erozan YS, Askin F, Sidransky D. Microsatellite alterations as clonal markers for the detection of human cancer. Proc Natl Acad Sci U S A. 1994;91:9871–9875. doi: 10.1073/pnas.91.21.9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlegel J, Bocker T, Zirngibl H, Hofstädter F, Rüschoff J. Detection of microsatellite instability in human colorectal carcinomas using a non-radioactive PCR-based screening technique. Virchows Arch. 1995;426:223–227. doi: 10.1007/BF00191358. [DOI] [PubMed] [Google Scholar]
- 10.Lothe RA. Microsatellite instability in human solid tumors. Mol Med Today. 1997;3:61–68. doi: 10.1016/S1357-4310(96)10055-1. [DOI] [PubMed] [Google Scholar]
- 11.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 12.Christensen M, Jensen MA, Wolf H, Orntoft TF. Pronounced microsatellite instability in transitional cell carcinomas from young patients with bladder cancer. Int J Cancer. 1998;79:396–401. doi: 10.1002/(sici)1097-0215(19980821)79:4<396::aid-ijc15>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Berger AP, Parson W, Stenzl A, Steiner H, Bartsch G, Klocker H. Microsatellite alterations in human bladder cancer: detection of tumor cells in urine sediment and tumor tissue. Eur Urol. 2002;41:532–539. doi: 10.1016/s0302-2838(02)00073-8. [DOI] [PubMed] [Google Scholar]
- 14.Catto JW, Azzouzi AR, Amira N, Rehman I, Feeley KM, Cross SS, et al. Distinct patterns of microsatellite instability are seen in tumours of the urinary tract. Oncogene. 2003;22:8699–8706. doi: 10.1038/sj.onc.1206964. [DOI] [PubMed] [Google Scholar]
- 15.Sauter G, Algaba F, Amin M, Busch C, Cheville J, Gasser I, et al. Non-invasive urothelial tumours. Eble JN, Sauter G, Epstein JI, Sesterhenn IA (eds). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. IARC Press, Lyon. 2004:110–112. [Google Scholar]
- 16.Bonnal C, Ravery V, Toublanc M, Bertrand G, Boccon-Gibod L, Henin D, et al. Absence of microsatellite instability in transitional cell carcinoma of the bladder. Urology. 2000;55:287–291. doi: 10.1016/s0090-4295(99)00399-4. [DOI] [PubMed] [Google Scholar]
- 17.Vaish M, Mandhani A, Mittal RD, Mittal B. Microsatellite instability as prognostic marker in bladder tumors: a clinical significance. BMC Urol. 2005;5:2. doi: 10.1186/1471-2490-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinicrope FA, Sargent DJ. Molecular pathways: microsatellite instability in colorectal cancer: prognostic, predictive, and therapeutic implications. Clin Cancer Res. 2012;18:1506–1512. doi: 10.1158/1078-0432.CCR-11-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loeb LA. Microsatellite instability: marker of a mutator phenotype in cancer. Cancer Res. 1994;54:5059–5063. [PubMed] [Google Scholar]
- 20.Gleeson CM, Sloan JM, McGuigan JA, Ritchie AJ, Weber JL, Russell SE. Ubiquitous somatic alterations at microsatellite alleles occur infrequently in Barrett’s-associated esophageal adenocarcinoma. Cancer Res. 1996;56:259–263. [PubMed] [Google Scholar]
- 21.Thiagalingam S, Foy RL, Cheng KH, Lee HJ, Thiagalingam A, Ponte JF. Loss of heterozygosity as a predictor to map tumor suppressor genes in cancer: molecular basis of its occurrence. Curr Opin Oncol. 2002;14:65–72. doi: 10.1097/00001622-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Nascimento e Pontes MG, da Silveira SM, Trindade Filho JC, Rogatto SR, Viana de Camargo JL. Chromosomal imbalances in successive moments of human bladder urothelial carcinoma. Urol Oncol. 2013;3:827–835. doi: 10.1016/j.urolonc.2011.05.015. [DOI] [PubMed] [Google Scholar]


