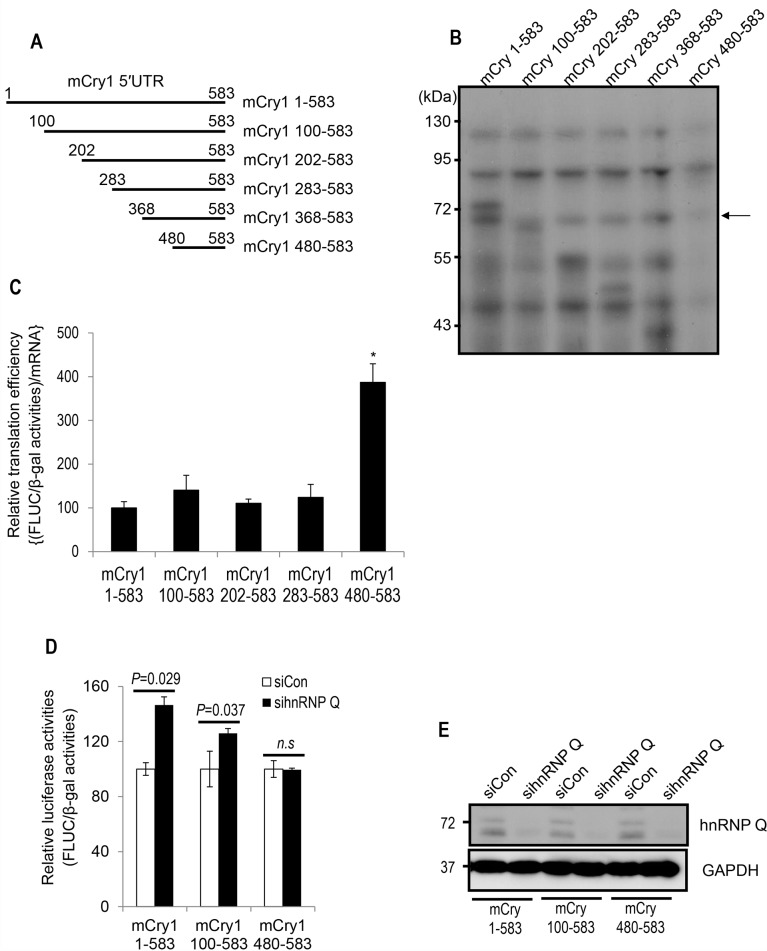
Fig 2. The cis-acting region for hnRNP Q resides in the forepart of the mCry1 5′UTR.
(A) Schematic description of serially deleted mCry1 5′UTRs. (B) Cellular proteins that bound to the full-length or truncated forms of the mCry1 5′UTR were analyzed by in vitro binding followed by UV-crosslinking assays. The arrow indicates the bands corresponding to hnRNP Q. (C) The translation efficiency of the full-length or deleted forms of the mCry1 5′UTR was determined. Transfection consistency was compensated with β-Gal activity, and translation efficiency was further calculated with mRNA amount derived from each construct. Error bars represent the SEM of four independent experiments. P-value of mCry1 1–583 vs mCry1 480–583 = 0.032. P-value of mCry1 100–583 vs mCry1 480–583 = 0.048. P-value of mCry1 202–583 vs mCry1 480–583 = 0.026. P-value of mCry1 283–583 vs mCry1 480–583 = 0.036. (D) The enhancement of translation mediated by the full-length or deleted forms of the mCry1 5′UTR under hnRNP Q silencing is shown. Error bars represent the SEM of three independent experiments. (E) Downregulation of hnRNP Q was confirmed by immunoblotting.