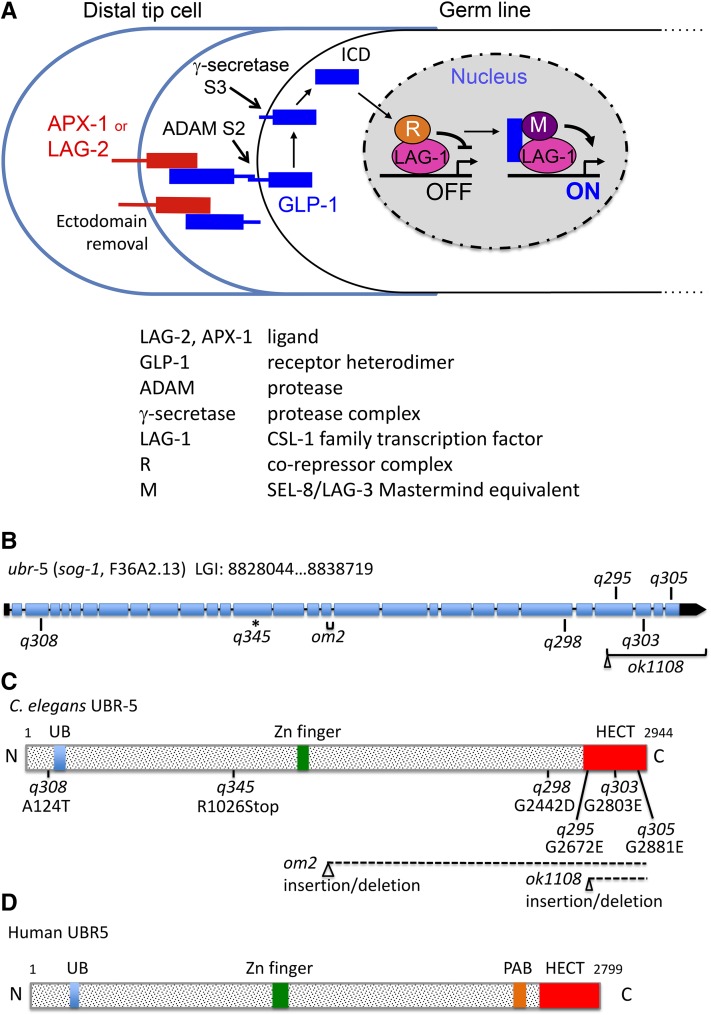
Figure 1.
sog-1 encodes UBR-5, a HECT-type E3 ubiquitin ligase. (A) Working model for GLP-1/Notch signaling in the adult C. elegans germline. Interaction of transmembrane LAG-2 and/or APX-1 ligand and GLP-1 heterodimer triggers proteolytic cleavage of GLP-1. The S2 cleavage requires an ADAM family protease and releases the GLP-1 ectodomain (bound to LAG-2); the S3 cleavage requires γ-secretase and releases the GLP-1 intracellular domain (ICD) for transport to the nucleus. Nuclear GLP-1 ICD interacts with the CSL-1-type transcription factor, LAG-1, and the coactivator SEL-8/LAG-3 (M), and displaces the LAG-1-bound corepressor complex (R). Signaling “strength” is modulated by numerous processes, as described in the text. (B) Diagram represents ubr-5 gene structure; mutant lesions associated with suppression of glp-1 are indicated. Nucleotide coordinates refer to genome version WS240. (C) Diagram represents UBR-5 protein structure. Conserved domains are indicated, as are amino acid substitutions and deletions/insertions associated with mutant alleles. The UB domain is variably referred to as the EDD or E3 domain in the literature. See Table S2 for details of sequence insertions and deletions in ok1108 and om2. (D) Domain architecture of human UBR5. Many UBR5 family members contain a poly(A) binding protein (PABP) motif (aka MLLE motif) just upstream of the HECT domain.