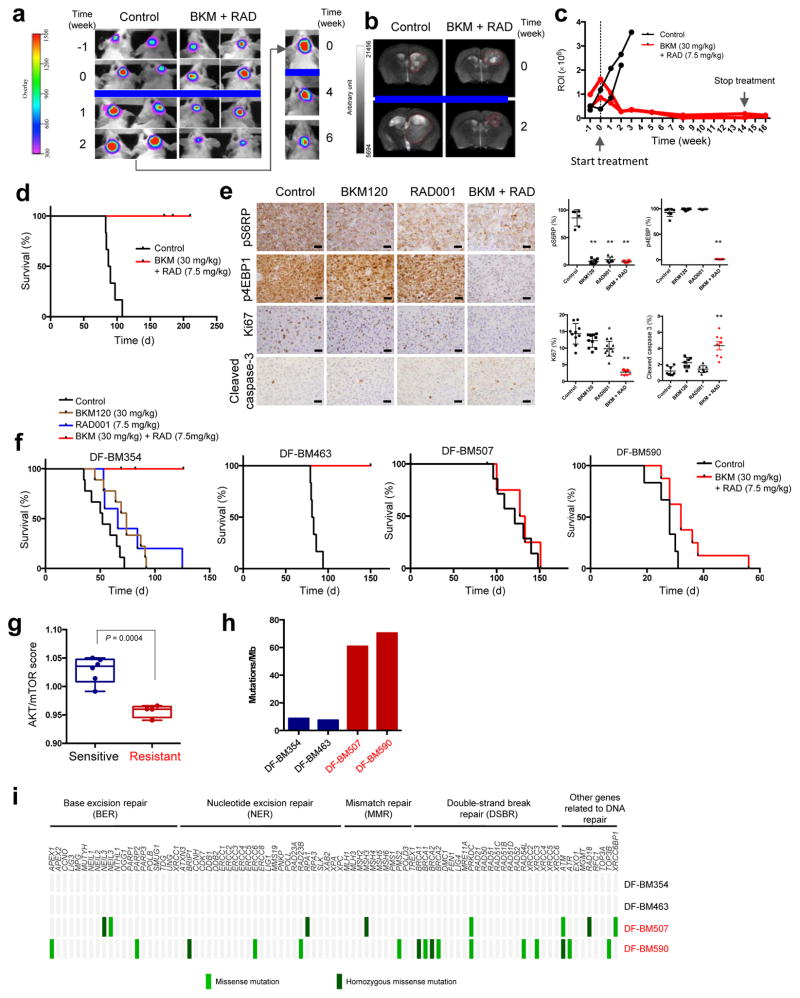
Figure 2. Differential responses of HER2-positive BCBM PDXs to the combination of BKM120/RAD001.
(a) Representative bioluminescence imaging analysis of mice bearing DF-BM355 tumor before and after treatment with combined BKM120 (30 mg/kg) and RAD001 (7.5 mg/kg), n = 5. (b) Representative MRI of DF-BM355-bearing mice treated with vehicle control or combined BKM120 with RAD001, n = 3. (c) Quantification of the regions of interest (ROI) determined at each imaging time point, n = 2. (d) Kaplan–Meier survival of DF-BM355-bearing mice treated with vehicle control or BKM120 + RAD001, n = 6. (e) IHC analyses of p-4EBP1, p-S6RP, Ki67 and cleaved caspase-3 on DF-BM355 tumors treated for 4 days with indicated treatments (Scale bars = 25 μm). Graphs represent mean ± s.d. (n = 6–10 images per group, *P < 0.05, **P < 0.01, one-way ANOVA followed by Dunnett’s test). (f) Kaplan–Meier survival of mice bearing DF-BM354, DF-BM463, DF-BM507, and DF-BM590 with vehicle control or compound as indicated. n = 5–9. (g) Transcriptome analysis of AKT/mTOR-dependent signature genes from brain xenograft tumor tissues from untreated mice. Boxplots correspond to the first and third quartiles with upper and lower whisker extending to the highest value that is within 1.5 times the interquartile range (n = 4–6 per group, P = 0.0004, Student’s t-test). (h) Number of somatic mutations in HER2-positive BCBM PDXs identified by WES. (i) Mutational profiling of a panel of DNA repair genes.