Abstract
Zidovudine (AZT) remains the mainstay of antiretroviral therapy against HIV in resource-poor countries; however, its use is frequently associated with hepatotoxicity. Not all HIV patients on AZT develop hepatotoxicity, and the determining factors are unclear. Alcohol consumption and cigarette smoking are known risk factors for HIV hepatotoxicity, and both are significant sources of acrolein, a highly reactive and toxic aldehyde. This study examines the potential hepatotoxic interactions between acrolein and AZT. Our data demonstrate that acrolein markedly enhanced AZT-induced transcriptionally permissive histone modifications (H3K9Ac and H3K9Me3) allowing the recruitment of transcription factor NF-kB and RNA polymerase II at the FasL gene promoter, resulting in FasL upregulation and apoptosis in hepatocytes. Notably, the acrolein scavenger, hydralazine prevented these promoter-associated epigenetic changes and inhibited FasL upregulation and apoptosis induced by the combination of AZT and acrolein, as well as AZT alone. Our data strongly suggest that acrolein enhancement of promoter histone modifications and FasL upregulation are major pathogenic mechanisms driving AZT-induced hepatotoxicity. Moreover, these data also indicate the therapeutic potential of hydralazine in mitigating AZT hepatotoxicity.
Keywords: Zidovudine (AZT), Acrolein, FasL, Hepatocytes, Epigenetic promoter modifications, Hydralazine
Introduction
Hepatotoxicity is a significant problem for patients with Human Immunodeficiency Virus-1 (HIV) infection who are treated with antiretroviral therapy (ART), and it accounts for 14–18% of HIV patient deaths not associated with AIDS (Price and Thio, 2010). Thus, although ART has greatly reduced the morbidity and mortality in HIV infected patients, its toxic side effects remain a concern and lead to restriction of long-term use or treatment discontinuation (Bica et al., 2001; Nunez and Soriano, 2005; Palella et al., 2006; Woreta et al., 2011). Zidovudine (3-azido-39-deoxythymidine (AZT), a nucleoside reverse transcriptase inhibitor (NRTI), was the first FDA- approved drug for HIV patients, and continues to be the mainstay of HIV-ART in developing countries (Fang and Beland, 2009; Kalyesubula et al., 2011). Because of its efficacy, AZT is used alone or in combination for prevention of perinatal transmission and combined antepartum, intrapartum, and infant antiretroviral prophylaxis in resource-poor settings, as well as in the United States (Senise et al., 2011; Sturt et al., 2010; website, 2016). Hence, AZT remains a relevant constituent of anti-HIV therapy worldwide. However, long-term use of AZT is associated with significant hematologic disorders, cardiomyopathy and hepatotoxicity (Chariot et al., 1999; Desai et al., 2012; Szabados et al., 1999). With regard to hepatotoxicity, AZT is known to cause mitochondrial dysfunction and apoptosis, however, the detailed mechanisms are not completely understood (Acosta and Grimsley, 1999; Banerjee et al., 2013; Chariot et al., 1999; de la Asuncion et al., 1999; Freiman et al., 1993).
Notably, not all HIV-infected patients on ART develop liver toxicity; up to 20% have elevated liver enzymes and only 2–10% develop severe hepatic injury (Acosta and Grimsley, 1999; Johnson et al., 2001). The factors that determine susceptibility to ART hepatotoxicity are not clearly established; however environmental and dietary factors have the potential to contribute to liver injury. In this regard, cigarette smoking and alcohol consumption are the two common contributing risk factors that occur frequently in HIV infected individuals and are known to adversely influence their health (Lloyd-Richardson et al., 2008; Miguez et al., 2003). The rate of smoking is 2–3 times higher among HIV-positive adults compared to the general public, with high usage of 16–23 cigarettes per day (Benard et al., 2007; Lloyd-Richardson et al., 2008). Similarly, alcohol use is more frequent among HIV-infected persons with 50% reporting regular alcohol use, and half as many reporting excessive/hazardous alcohol consumption (Galvan et al., 2002). Both alcohol and smoking are independently known to decrease cellular antioxidant status, enhance oxidative stress and increase cellular lipid peroxidation (LPO) (Papadopoulou and Bloomer, 2007), and increased lipid peroxidation is documented in HIV-infected individuals (Teto et al., 2013; Vassimon et al., 2010). Acrolein is one of the most toxic and reactive aldehyde byproducts of lipid peroxidation (Moghe et al., 2015), and is a major aldehyde component of cigarette smoke (Cerami et al., 1997; Hristova et al., 2012; Muscat et al., 1991). Thus, the two common comorbidities in HIV are known sources of acrolein. Reported or calculated levels of acrolein exposure and/or generation vary widely and it is difficult to extrapolate the acrolein concentrations that may be relevant in the liver. Studies have shown that higher acrolein metabolite levels ranging from approximately 30μM to 180 μM are detected in body fluids under pathological conditions (Carmella et al., 2007; Eiserich et al., 1995; Calingasan et al., 1999; Lovell and Markesbery, 2001; Sakata et al., 2003; Satoh et al., 1999; Tsukahara et al., 2002). Notably, our earlier studies have examined acrolein toxicity (5μM to 100μM) in hepatocytes, and shown that acrolein has multiple adverse effects in hepatocytes (such as GSH depletion, mitochondrial dysfunction and ER stress) which contribute to its toxicity (Mohammad et al., 2012). However, the interactions of acrolein and ART have not been studied, and it is unclear whether acrolein exposure predisposes to or potentiates ART hepatotoxicity.
The role of the Fas-FasL signaling pathway in the apoptotic death of hepatocytes has been demonstrated in several forms of liver disease, particularly drug-induced liver injury (Feldstein et al., 2003; Malhi et al., 2010; Pianko et al., 2001). Typically, hepatocytes express high levels of Fas receptor and respond to FasL signals from activated immune cells; however, under certain conditions, hepatocytes also express high levels of FasL, leading to fratricide and liver injury (Galle et al., 1995). With regards to HIV-positive individuals, increased expression of FasL is seen in cardiomyocytes, which contributes to AZT-Induced cardiomyopathy (Purevjav et al., 2007). However, the role of FasL expression and apoptotic death in AZT induced hepatotoxicity has not been examined. We and others have shown that promoter-associated epigenetic modifications are known to regulate FasL gene expression (Castellano et al., 2006; Ghare et al., 2014; Holtz-Heppelmann et al., 1998; Li-Weber and Krammer, 2003). Hence, the present study examines the potential hepatotoxic interactions between acrolein and the ART drug, AZT. Specifically, we investigated the effect of acrolein and AZT on the epigenetic regulation of FasL, and consequent FasL-mediated apoptotic death of hepatocytes.
Materials and Methods
Cell culture
HepG2 cells (clone E6-1) obtained from ATCC (ATCC, Rockville, MD) were cultured in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 10 U/ml penicillin and 10 μg/ml streptomycin. Cells were maintained in an incubator at 37°C with humidified 5% CO2 and used in passages 3–7. Cells were plated (at 0.25 million cells per ml) and incubated overnight in media containing 10% FBS. In all experiments, treatments were done in serum-free media.
Primary rat hepatocytes were obtained from TRL (Triangle Research Laboratory, NC) and maintained in accordance with company instructions. For experiments, cells were plated (at 0.25 million cells per ml) on non-collagen coated plates (to maintain ACR reactivity) and kept in an incubator at 37°C with humidified 5% CO2 overnight. Treatments for all experiments were done in serum free Dulbecco’s modified eagle medium (DMEM) supplemented with 10 U/ml penicillin and 10 μg/ml streptomycin.
Reagents
AZT, acrolein and hydralazine were purchased from Sigma-Aldrich (St. Louis, MO). Fetal bovine serum was purchased from Atlanta Biologicals (Norcross, GA). DMEM, penicillin, streptomycin and TRIzol® were obtained from Invitrogen (Carlsbad, CA). Caspase-8 inhibitor Z-IETD-FMK was purchased from R&D Systems, Inc. (Minneapolis, MN). Antibodies for FasL, caspase 8, caspase 3, and GAPDH were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Treatments
AZT and hydralazine stock solutions were made in water and stored as per the company instructions. Acrolein was made in serum free media and immediately used for the experimental treatments. Caspase-8 inhibitor was dissolved in DMSO to yield a concentrated stock solution, such that final concentration of DMSO in the treatment will be less than 0.1%. Treatment with DMSO alone served as a negative solvent control (data not shown).
The final concentrations used in the study were 100μM and 250μM for AZT and 12.5μM and 25μM for acrolein. These concentrations were selected based upon the results from preliminary dose response experiments (data not shown) and published literature (Fang and Beland, 2009; Fang et al., 2014; Mohammad et al., 2012; Sun et al., 2014).
Cell Viability-MTT assay
Cell survival/cell death was measured in treated cells by the MTT (3, (4, 5-dimethylthiazol-2-yl) 2, 5-diphenyltetrazolium bromide) assay as described previously (Mohammad et al., 2012).
DNA fragmentation ELISA
DNA fragmentation was measured using a Cell Death Detection ELISA kit (Roche Applied Sciences, Indianapolis, IN) in accordance with manufacturer’s instructions (Mohammad et al., 2012).
RNA isolation and real time PCR analysis
Total RNA was isolated from cells using TRIzol® (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. For real-time PCR, the first-strand cDNA was synthesized using qScriptTM cDNA SuperMix (Quanta Biosciences, Inc., Gaithersburg, MD) using 100 ng of total RNA. The RT conditions were 10 min at 25°C, 30 min at 48°C and 5 min at 85°C. Real time PCR was performed with an ABI prism 7500 sequence detection system and PerfeCtaTM SYBR Green FastMixTM, Low ROX reagents (Quanta Biosciences, Inc.). Each sample was run in duplicate or triplicate reactions as technical replicates. Reverse transcriptase polymerase chain reaction (RT-PCR) assays were used to assess mRNA levels. Several housekeeping genes (β-actin, TATA binding protein, and 18S rRNA) were initially used for normalization and optimization, and no significant differences were seen. For the manuscript, only data normalized with 18S rRNA is shown. FasL and 18S rRNA specific primers were designed in the lab and synthesized by Integrated DNA Technologies, Inc. (Coralville, IA).
Primers used were as follows: FasL-FP 5′-TCTACCAGCCAGATGCACAC-3′, FasL-RP 5′-CAGAGGCATGGACCTTGAGT-3′. 18s-FP 5′-CTCAACACGGGAAACCTCAC-3′, 18s-RP 5′-CGCTCCACCAACTAAGAAC-3′. cycle number at which the The parameter threshold cycle (Ct) was defined as the fraction fluorescence passed the threshold. The gene expression was analyzed by relative quantification using 2–ΔΔCt method by normalizing with 18s rRNA and data is presented as fold change over UT which is set at 1.
Western blot analysis
Total cellular extracts were prepared using RIPA lysis buffer (25mM Tris-HCl pH 7.5,150mM NaCl, 1% NP-40, 0.1% SDS, 1mM DTT, 10% glycerol, 1x protease inhibitor cocktail, 0.5% sodium deoxycholate, 1mM Na2VO3 and 10mM NaF). Protein samples (40μg) were prepared in SDS loading buffer and proteins were separated by electrophoresis on pre-cast 4 –15% gradient polyacrylamide gels and transferred onto a PVDF membrane. The blots were blocked with 5% (w v) nonfat dry milk constituted in 1x TBS-T (Tris buffered saline, 10 mM Tris-HCl pH 8.0, 150 mM NaCl, 0.1% Tween 20) for 1 hour at room temperature. Membranes were incubated at 4°C overnight with primary antibodies directed against protein-of-interest in 5% (w v) nonfat dry milk in 1x TBS-T. After overnight incubation in primary antibody, blots were washed and incubated with appropriate horseradish peroxidase-conjugated secondary antibodies for an hour. Immunoreactive bands were visualized using the enhanced chemiluminescence light (ECL) detection reagents (Amersham, Arlington Heights, IL). Detection of GAPDH served as a loading control. Quantification was performed with Scion Image analysis software (Scion, Frederick, MD) (Ghare et al., 2011). Data shown are representative of 3 independent experiments.
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was conducted using the ChIP assay kit according to the manufacturer’s instructions (Millipore, Billerica, MA). Briefly, 10 × 106 cells were crosslinked with 1% formaldehyde- 1 X-glycine and lysed in lysis buffer containing protease inhibitor cocktail. Enzymatic digestion of DNA was performed as per manufacturer’s protocol (EZ-Zyme Magna CHIP KIT) to produce chromatin fragments of 100–300 bp, which were confirmed 1% agarose gel electrophoresis. ChIP antibodies directed against protein of choice were used for immunoprecipitation. Anti-trimethyl-histone H3 Lys 4 (H3K4Me3), anti-acetyl-histone H3 Lys 9 (H3K9Ac), NF-kB (p65), and RNA Polymerase II (RNA POL-II) were from Upstate Biotechnology (Lake Placid, NY) and a non-specific control rabbit and mouse IgG were from Cell Signaling Technology, Beverly, MA. The chromatin lysates were incubated overnight with antibody, following which the antibody/protein complexes were isolated using magnetic beads. After reverse crosslinking and deproteinization, DNA was purified by column purification (MOBIO, Carlsbad, CA). ChIP-qPCR was performed using primers specific for different regions of the FasL promoter on the ABI Prism 7500 Sequence Detection System as described (Ghare et al., 2014; Gobejishvili et al., 2011). Data was analyzed as Differential Occupancy Fold Change. ChIP-qPCR results were calculated by ΔΔCt method where each ChIP DNA Ct value was normalized to the Input DNA Ct value using ΔCt [normalized ChIP] = (Ct [ChIP] - (Ct [Input] - Log2 (Input Dilution Factor))). The difference between the normalized experimental sample (S2) and the control sample (S1) ChIP fraction Ct values (second ΔΔCt) was determined using ΔΔCt [S2–S1] = ΔCt [S2: normalized ChIP] - ΔCt [S1: normalized ChIP].
Statistical analysis
Data are presented as mean ± SEM for the indicated number of independently performed experiments. One-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test was used and the differences were defined as significant at p<0.05. Graph Pad Prism 5 software (version 5.03) was used to analyze all data sets.
Results
The objective of this research was to investigate the combined effects of acrolein (ACR) and the ART drug, AZT, on hepatotoxicity with a particular focus on FasL expression and FasL-mediated apoptotic death. We used a well-characterized cultured cell system, (human hepatoma cell line, HepG2), along with primary rat hepatocytes to confirm the key findings. HepG2 cells represent a well-accepted model system for hepatocytes, and the cells retain a majority of the characteristics of normal hepatocytes including signal transduction pathways, metabolic features, and stress/death responses. Moreover, HepG2 cells have been extensively used in numerous toxicity and other liver-related studies by us and others (Fang et al., 2014; Mohammad et al., 2012). Additionally, we have repeated critical experiments in primary rat hepatocytes to ensure that our findings were not limited to the transformed phenotype of HepG2 cells.
Acrolein enhances AZT-induced apoptotic death in hepatocytes
To determine whether exposure to acrolein sensitizes hepatocytes to AZT-induced cell death, we examined the combined effects of acrolein and AZT on cell viability by the MTT assay. Cultured human hepatocytes (HepG2 cells) were treated with AZT (100 and 250μM) and ACR (12.5, 25 and 50μM), either singly or in combination, for 48hr. AZT treatment by itself was not toxic at both concentrations. In comparison, ACR did not induce cell death at 12.5 μM but reduced survival at the higher concentrations. Notably, the data showed that AZT and ACR together caused significant hepatocyte death even at sub toxic individual concentrations (Fig. 1A). To validate the findings seen in cultured cells, we examined the cytotoxic effects of combinatorial exposure of acrolein and AZT in primary rat hepatocytes treated with ACR (12.5 and 25μM) and AZT (100 and 250μM). Similar to HepG2 cells, AZT+ACR together increased cell death in primary hepatocytes (Fig. 1B). Since the purpose of this study is to better understand the potential interactions of acrolein and AZT in the development of hepatotoxicity, we used 250 μM AZT and 12.5 μM ACR for further analysis.
Figure 1. Acrolein enhances AZT- induced apoptotic cell death.
Cells were untreated (UT) or treated with AZT (100 and 250 μM) and acrolein (12.5 and 25 μM) individually or in combination at varying concentrations (as indicated) for 48h. Cell viability was determined by MTT assay in HepG2 cells (1A) and in rat primary hepatocytes (1B). The data were normalized to untreated values (set to 100). Results are represented as mean ± SEM (n=3, independent experiments). Apoptosis was analyzed by DNA Fragmentation ELISA in HepG2 cells (1C) and in rat primary hepatocytes (1D). Data are expressed as fold over UT from three separate experiments. Results are represented as mean ± SEM (n=3, independent experiments). Statistical analysis was performed by one-way ANOVA with Bonferroni’s correction for multiple comparisons. P values: p <0.05; a = compared with UT, b = compared with corresponding AZT, and c = compared with corresponding ACR.
Apoptosis is a key mechanism involved in hepatocyte cell death. Hence, we assessed apoptotic cell death by measuring DNA fragmentation, a hallmark of apoptosis. Both HepG2 cells and primary hepatocytes were treated with ACR (12.5μM) and AZT (250μM) alone or in combination for 48h. DNA fragmentation was quantified using cell death detection ELISA (Fig. 1C). Similar to the MTT results, the combinatorial treatment significantly increased apoptotic cell death as compared to untreated and single treatments we observed marginal increase in DNA fragmentation with both AZT and ACR in (Fig. 1C and D). Thus, our data showed that acrolein exposure enhanced AZT-induced apoptosis in hepatocytes.
Apoptotic cell death induced by the combination of AZT and ACR is mediated by Fas-FasL signaling pathway
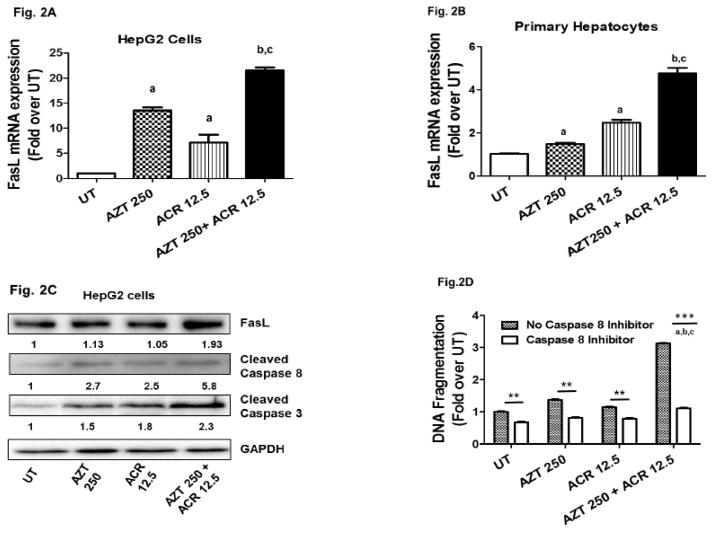
The role of FasL- Fas receptor signaling is well established in the apoptotic death of hepatocytes. Hence, we examined the key components of the FasL-Fas pathway in the hepatotoxic response to AZT and ACR. Since FasL is the initiator protein of the Fas-FasL apoptotic pathway, we initially analyzed FasL mRNA expression. HepG2 cells and primary rat hepatocytes were treated as detailed above. Treatment with a combination of AZT+ACR resulted in a significant induction in FasL mRNA expression in both HepG2 cells and primary hepatocytes (Fig. 2A & 2B). Notably, individual treatments with AZT and ACR were also able to induce FasL mRNA expression, albeit at a relatively lesser extent than the combination. Further, the increase in FasL mRNA was accompanied by a consequent increase in the protein level of FasL (Fig. 2C).
Figure 2. Acrolein upregulates FasL expression and enhances FasL-Fas mediated apoptotic signaling.
Cells were untreated (UT) or treated with AZT (AZT 250μM) and acrolein (ACR 12.5μM) individually or in combination for 48h. FasL mRNA levels were determined by real time PCR and expressed as fold over UT in HepG2 cells (2A) and in primary rat hepatocytes (2B). Data are represented as mean ± SEM (n=3, independent experiments). Statistical analysis was performed by one-way ANOVA with Bonferroni’s correction for multiple comparisons. P values: p<0.05; a = compared with UT, b = compared with AZT, and c = compared with ACR. (2C) Total HepG2 cell lysates (48 h) were analyzed for FasL, active Caspase 8 and active Caspase 3 protein levels by Western blot analysis with GAPDH as loading control. A representative of 3 separate experiments is shown. Numbers represent densitometry ratios using GAPDH as control. (2D) DNA fragmentation analysis was performed in HepG2 cells treated with Caspase 8 inhibitor (50μM) 1h prior to each respective treatment and the data was shown with (clear bar) or without (pattern bar) Caspase-8 inhibitor. Results are represented as mean ± SEM (n=3, independent experiments), with one-way ANOVA and Bonferroni’s correction for multiple comparisons posttest. P values: p<0.05; a = compared with UT, b = compared with AZT and c = compared with ACR, and **p < 0.01 and ***p< 0.001 between indicated treatments.
Subsequent to the induction of FasL gene expression, we examined the involvement of FasL apoptotic signaling components. Specifically, the effect of AZT and ACR on the activation of caspases-8 and -3 was examined in the context of FasL expression and apoptotic death. Proteolytic activation of caspases-8 and -3 into their active cleaved forms was examined by Western blot analysis using total HepG2 cell lysates. Commensurate with FasL expression, maximal increase in the active cleaved forms of caspase 8 and caspase 3 was observed with the combination of ACR+AZT (Fig. 2C).
Proteolytic activation of the initiator caspase-8 is an early event triggered by FasL-Fas interactions, leading to activation of the executioner/effector caspase-3, and consequent FasL-mediated apoptosis. Hence, the contribution of FasL- and caspase-8 mediated apoptotic signaling in AZT+ACR induced death in hepatocytes was evaluated. HepG2 cells were pretreated with a caspase-8 specific inhibitor (50μM) for an hour before exposing them to ACR and AZT. After 48 hours of treatment, apoptotic cell death was assessed by DNA fragmentation. Our results showed that treatment with caspase 8 inhibitor significantly abrogated DNA fragmentation induced by all treatments (AZT, ACR, and AZT+ACR) (Fig. 2D).
Combination of Acrolein and AZT induces epigenetic modifications at the FasL promoter
Our recent work has demonstrated that FasL gene transcription is critically controlled by coordinate promoter histone modifications (Ghare et al., 2014). Since, promoter histone modifications leading to transcriptionally permissive chromatin configuration are a prerequisite and precede mRNA expression, earlier time points are better suited to studying epigenetic modifications. The expression of FasL (and apoptotic effects) were observed at 48h, hence we chose an earlier time point of 24h to examine key epigenetic histone modifications. The proximal FasL promoter histone modifications were examined in specific I, II, III and IV regions as shown in the schematic (Fig. 3A). Regions I to III were chosen based on binding sites of relevant transcription factors that are known to regulate FasL gene transcription. The region IV (coding region) was examined as an internal negative control.
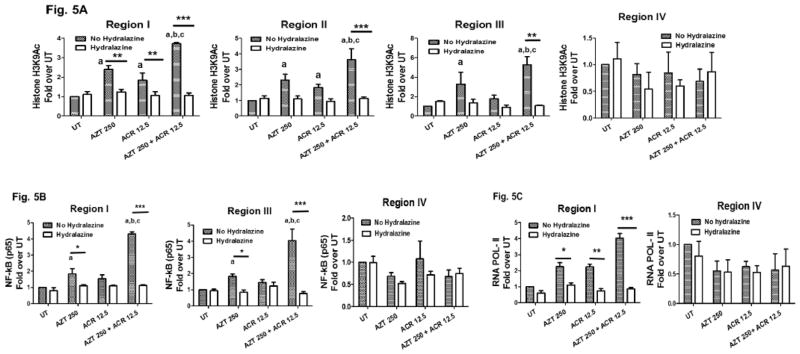
Figure 3. Acrolein enhances AZT- induced transcriptionally permissive histone modifications at FasL promoter.
(3A) Schematic of FasL promoter showing locations of ChIP-PCR primer pairs for analysis of epigenetic modifications; denoted as regions I–IV.TSR = Transcriptional start region and UTR = untranslated region. The coordinate locations shown are with respect to the transcription start site in REFSEQ NM_000639.1. The table shows primer sequences for each region and relevant transcription factor binding sites. ChIP-qPCR analysis of epigenetic modifications at FasL promoter was performed in HepG2 cells that were untreated (UT) or treated with AZT (AZT 250μM) and acrolein (ACR 12.5μM) individually or in combination for 24h. Histone modifications were assessed by analyzing chromatin that was immunoprecipitated with anti-trimethylated Histone H3 lysine 4 (H3K4Me3) (3B) and anti-acetylated Histone H3 lysine 9 (H3K9Ac) (3C) antibodies. Levels of histone modifications were measured using primer for regions I–IV shown in the schematic. Differences are expressed as fold over UT after normalizing for input DNA. Results are represented as mean ± SEM (n=3, independent experiments). Statistical analysis was performed by one-way ANOVA with Bonferroni’s correction for multiple comparisons. P values: p<0.05; a = compared with UT, b = compared with AZT, and c = compared with ACR.
Acrolein and AZT exposure increases transcriptionally permissive histone H3 modifications at the FasL promoter
We have recently demonstrated that transcriptionally permissive histone modifications, histone 3 lysine 4 trimethylation (H3K4Me3) and histone 3 lysine 9 acetylation (H3K9Ac) play a critical role in the transcriptional activation of FasL in CD4+ T lymphocytes (Ghare et al., 2014). Chromatin was obtained from HepG2 cells treated either singly or in combination with ACR (12.5μM) and AZT (250μM) for 24h, and ChIP analysis was performed using antibodies that selectively recognize H3K4 trimethylation and H3K9 acetylation. Correspondent with the observed FasL gene expression, the combination of acrolein and AZT maximally increased the transcriptionally permissive histone H3 modifications in the three relevant promoter regions (Fig. 3B and 3C). Notably, AZT alone also increased H3K4Me3 and H3K9Ac levels, albeit to a lesser degree as compared to the combination; whereas acrolein alone did not affect H3K4Me3 levels, but did increase H3K9Ac levels. Importantly, in comparison to the three promoter regions, region IV located at the 3′ end of the FasL gene, did not show any significant changes with both H3K4Me3 and H3K9Ac levels in response to acrolein and AZT treatments.
Acrolein- and AZT-induced histone modifications allowed the recruitment of relevant transcription factors and RNA polymerase II (RNA Pol- II) to the FasL promoter
Since NF-kB (p65) is known to be an essential transcription factor for FasL gene expression in hepatocytes (Kavurma and Khachigian, 2003), we next examined the recruitment of NF-kB (p65) to regions I and III in the FasL promoter by ChIP analysis (Fig. 4A). Commensurate with the increase in H3K4Me3 and H3K9Ac and FasL gene expression, hepatocytes treated with acrolein and AZT either individually or in combination also showed proportional increase in NF-kB promoter binding (Fig. 4A). The ACR and AZT induced increase in the transcriptionally permissive FasL promoter changes and NF-kB recruitment were associated with a significant increase in the binding of RNA Pol-II at Region I, which encompasses the transcription start region (Fig. 4B).
Figure 4. Acrolein and AZT-induced permissive histone modifications facilitate recruitment of transcription factors and RNA Pol II at the FasL promoter.
HepG2 cells were untreated (UT) or treated with AZT (AZT 250μM) and acrolein (ACR 12.5μM) individually or in combination for 24h. ChIP-qPCR analysis was performed to examine the recruitment of (4A) transcription factor NFAT at regions I, III and IV; and (4B) RNA Pol -II at regions I and IV of FasL promoter. Differences are expressed as fold over UT after normalizing for input DNA. Results are represented as mean ± SEM (n=3, independent experiments). Statistical analysis was performed by one-way ANOVA with Bonferroni’s correction for multiple comparisons. P values: p<0.05; a = compared with UT, b = compared with AZT, and c = compared with ACR.
Overall, these data indicate that acrolein potentiated/amplified the effects of AZT to form an active, transcriptionally permissive state at the promoter leading to increased FasL expression and FasL-mediated apoptotic hepatocyte death.
Acrolein scavenger, hydralazine, prevents FasL gene expression and hepatocyte apoptosis induced by the combination of Acrolein and AZT
Hydralazine is a known scavenger of acrolein and acrolein-protein adducts, and shows protection against acrolein-induced neuronal cell death and spinal cord injury (Liu-Snyder et al., 2006). Hence, the effects of hydralazine in attenuating acrolein-mediated cellular events that enhance AZT cytotoxicity were examined. We examined the effect of hydralazine on the FasL promoter-histone modifications that occur in response to treatment with acrolein and AZT. HepG2 cells were pretreated with hydralazine (100μM) for 1h and rinsed with serum free media to remove any extracellular hydralazine. Subsequently, the cells were exposed to ACR (12.5μM) and AZT (250μM), both singly and in combination as mentioned above. ChIP analysis showed that hydralazine prevented the transcription-activating histone modification, H3K9Ac, induced by acrolein and AZT (Fig. 5A). Consequently, hydralazine also prevented the recruitment of NF-kB and RNA pol II to the FasL promoter (Fig. 5B and 5C).
Figure 5. Hydralazine attenuates ACR+AZT- induced transcriptionally permissive epigenetic modifications at FasL promoter.
HepG2 cells were untreated or pretreated with hydralazine (100μM, 1hr) followed by AZT (AZT 250μM) and acrolein (ACR 12.5μM) treatment individually or in combination for 24h. ChIP-qPCR quantification from anti Histone H3K9 acetylation (5A), anti-NF-kB (5B) and anti- RNA Pol II (5C) immunoprecipitated chromatin was performed. Data was shown as bar graph for hydralazine treated (Clear bar) or non-treated (pattern bar) groups. Differences are expressed as fold over UT after normalizing for input DNA. Results are represented as mean ± SEM (n=3, independent experiments). Statistical analysis was performed by one-way ANOVA with Bonferroni’s correction for multiple comparisons. P values: p<0.05; a = compared with UT, b = compared with AZT, and c = compared with ACR, and **p < 0.01 and ***p< 0.001 between indicated treatments. Filled bars: No hydralazine and Unfilled bars: Hydralazine.
Further, we also examined the effects of hydralazine on acrolein and AZT induced FasL gene expression and apoptotic death in HepG2 cells as well as primary rat hepatocytes. Commensurate with the prevention of FasL promoter associated transcription activating epigenetic changes, pretreatment of hepatocytes with hydralazine also inhibited FasL mRNA expression (Fig. 6A) and apoptotic death (Fig. 6B) induced by acrolein and AZT. Thus our data showed that the acrolein scavenger hydralazine protected hepatocytes from cytotoxic effects induced by ACR and AZT.
Figure 6. Hydralazine, an acrolein scavenger, abrogates ACR+AZT- induced FasL gene expression and apoptotic hepatocyte death.
Effect of acrolein scavenging was examined by treating hepatocytes with hydralazine (100μM, 1hr) followed by AZT (AZT 250μM) and acrolein (ACR 12.5μM) treatment individually or in combination. Real time PCR analysis for FasL mRNA (6A) and DNA fragmentation apoptosis assay (6B) was performed in HepG2 cells (left panel) and in rat primary hepatocytes (right panel). Results are represented as mean ± SEM (n=3, independent experiments). Statistical analysis was performed by one-way ANOVA with Bonferroni’s correction for multiple comparisons. P values: p<0.05; a = compared with UT, b = compared with AZT, and c = compared with ACR, and **p < 0.01 and ***p< 0.001 between indicated treatments. Filled bars: No hydralazine and Unfilled bars: Hydralazine.
Taken together, the data showed that acrolein enhances AZT-induced hepato-cytotoxicity via FasL promoter associated epigenetic changes and upregulation of FasL. These findings suggest that acrolein may be a relevant risk factor in the development of HAART-hepatotoxicity in HIV infected individuals. Additionally, the data also indicate that HAART hepatotoxicity may be therapeutically targeted by inactivating reactive aldehydes like acrolein.
Discussion
AZT continues to be a commonly prescribed anti-HIV drug worldwide, especially in resource poor regions (Kalyesubula et al., 2011; McGowan and Shah, 2000), and it is particularly effective during pregnancy to reduce the rate of HIV-1 transmission from mother to baby (Senise et al., 2011; Sturt et al., 2010). AZT usage is associated with hepatotoxicity, but not all HIV patients on AZT get liver injury; hence, understanding the mechanisms and factors that determine AZT toxicity remains relevant. Common sources of acrolein such as cigarette smoking and alcohol are known risk factors for hepatotoxicity. Additionally, acrolein is generated endogenously via lipid peroxidation, which is known to be elevated in HIV patients. Our study identifies acrolein as a potential cofactor that enhances AZT-induced hepatocyte death, and may act as a key determinant in the development of AZT hepatotoxicity.
We have examined the cumulative hepatotoxic interactions between AZT and acrolein, and the data showed that AZT hepatocyte toxicity was further compounded by exposure to acrolein. Notably, the AZT and acrolein-driven hepatotoxic mechanisms identified in HepG2 cells were also verified and validated in primary rat hepatocytes. Moreover, the reproducibility of the findings across species in human and rat cells, despite possible interspecies differences, adds scientific relevance to our study. The data demonstrate that FasL upregulation is a major component of AZT toxicity leading to hepatocyte apoptosis. The relevance of FasL-induced apoptotic signaling in hepatocyte toxicity was further established by the finding that caspase-8 inhibitor blocked ACR+AZT induced apoptosis. Numerous studies have shown that upregulation of either Fas receptor or FasL, or both, can trigger hepatocyte cell death following exposure to a variety of stimuli including organic pollutants, medicinal herbs, LPS-galactosamine, etc (Kuhla et al., 2015; Schattenberg et al., 2012; Zhang et al., 2015). In our study, induction of FasL expression and apoptotic signaling was a major component of AZT-induced hepatotoxicity. Further, there was minimal to no upregulation in Fas receptor at either mRNA or protein levels (data not shown) suggesting that AZT+ACR- induced FasL likely triggers Fas receptors in an autocrine and paracrine manner leading to death-receptor mediated hepatocyte suicide and fratricide as proposed by Galle et al (Galle et al., 1995; Kanzler and Galle, 2000; Muller et al., 1997). Although, other cell death modes may be involved, our data showing FasL expression, engagement of downstream apoptotic signaling and induction of DNA fragmentation strongly indicate that apoptotic death plays a predominant role in the hepatotoxic effects of AZT and acrolein. Significantly, the role of apoptosis is further reinforced by the finding that caspase inhibitor blocked AZT+ACR-induced DNA fragmentation and cell death.
Our work showed that distinct epigenetic mechanisms involving promoter histone modifications dictate the transcriptional activation and expression of FasL and subsequent apoptotic death in CD4 T lymphocytes (Ghare et al., 2014). To address the mechanisms underlying AZT+ACR-mediated enhancement in FasL expression in hepatocytes, we examined transcriptionally relevant histone modifications at the FasL promoter. AZT, both individually and in combination with acrolein, induced transcriptionally permissive histone modifications marked by H3K4Me3 and H3K9Ac at the FasL promoter. In relation to effecting transcription, increased promoter H3K4 tri-methylation is functionally correlated with an increase in H3K9 acetylation, a major modification of initiated and transcribed genes (Crump et al., 2011; Roh et al., 2006). Importantly, the degree of these histone modifications was significantly greater in hepatocytes exposed to a combination of AZT+ACR as compared to AZT and ACR alone; this observation corresponded to the lower cell death seen in the individual treatments. Further, these histone modifications were induced in the promoter regions known to regulate the access of transcription factors and transcriptional activation of FasL. The functional aspect of increased H3K4Me3 and H3K9Ac histone modifications involves the remodeling of chromatin and its de-compaction, making DNA more accessible to transcription factors and RNA polymerases (Grunstein, 1997; Liang et al., 2004; Roh et al., 2005). Indeed, consistent with the level of increase in these transcriptionally permissive modifications induced by AZT and ACR, either singly or in combination, there was a proportional increase in the level of NF-kB binding, RNA Pol-II recruitment which ultimately led to FasL gene expression.
Lastly, our data demonstrate the efficacy of hydralazine in mitigating acrolein and AZT induced epigenetic FasL upregulation and apoptosis, and provide proof-of principle for the therapeutic potential of hydralazine in ART hepatotoxicity. Further, hydralazine may have application in hepatotoxicity induced by other factors such as acetaminophen, alcohol, diet, and viruses where reactive aldehydes play an important role. Although hydralazine is shown to have a high affinity for acrolein, it is possible that hydralazine also neutralizes other reactive toxic aldehydes such as 4-hydroxynonenal that are known to be elevated in HIV infected individuals (Liu-Snyder et al., 2006; Sacktor et al., 2004). Interestingly, in addition to inhibiting the effects of the combination of AZT+ACR, hydralazine also blocked the baseline toxicity of AZT alone, suggesting that AZT-hepatotoxicity may be caused, at least in part, by the endogenous generation of toxic aldehydes such as acrolein.
Taken together, in relevance to ART hepatotoxicity, our study showed the involvement of transcriptionally permissive promoter histone modifications in acrolein and AZT driven FasL expression and hepatocyte cell death. Further, our study suggests that acrolein exposure through environmental, dietary or endogenous metabolic routes may be a significant determining factor in the development of ART hepatotoxicity in HIV infected individuals. Additionally, ART hepatotoxicity may be therapeutically targeted by acrolein/aldehydes scavengers.
Highlights.
Novel finding that AZT induces FasL expression leading to cell death in hepatocytes.
Dietary/environmental pollutant acrolein enhances AZT-induced FasL and apoptosis.
Epigenetic FasL promoter histone modifications are critical in upregulation by AZT+ACR.
Hydralazine prevents ACR+AZT-induced FasL expression and cytotoxicity.
Acknowledgments
Funding Information: This work was supported by the National Institutes of Health grants K01ES017105 (Joshi-Barve) AA024405 (Barve), ES014559 (McClain), AA022489 (McClain), AA021901 (McClain), AA021893 (McClain), AA023681 (McClain), AA018869 (McClain), the Department of Veterans Affairs (McClain), and the Department of Defense (McClain).
Abbreviations
- AZT
azidothymidine or Zidovudine
- ACR
acrolein
- ART
antiretroviral therapy
- ChIP
Chromatin immunoprecipitation
- FasL
Fas ligand
- H3K9Ac
Histone 3 lysine 9 acetylation
- H3K4Me3
Histone 3 lysine 4 trimethylation
- RNA Pol II
RNA polymerase II
- TF
Transcription Factor
- UTR
translational region
- TSR
Transcription start region
Footnotes
Conflict of interest and Disclosures:
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta BS, Grimsley EW. Zidovudine-associated type B lactic acidosis and hepatic steatosis in an HIV-infected patient. South Med J. 1999;92:421–423. doi: 10.1097/00007611-199904000-00015. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Abdelmegeed MA, Jang S, Song BJ. Zidovudine (AZT) and hepatic lipid accumulation: implication of inflammation, oxidative and endoplasmic reticulum stress mediators. PLoS One. 2013;8:e76850. doi: 10.1371/journal.pone.0076850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard A, Bonnet F, Tessier JF, Fossoux H, Dupon M, Mercie P, Ragnaud JM, Viallard JF, Dabis F, Chene G Groupe d’Epidemiologie Clinique du S.e.A. Tobacco addiction and HIV infection: toward the implementation of cessation programs. ANRS CO3 Aquitaine Cohort. AIDS Patient Care STDS. 2007;21:458–468. doi: 10.1089/apc.2006.0142. [DOI] [PubMed] [Google Scholar]
- Bica I, McGovern B, Dhar R, Stone D, McGowan K, Scheib R, Snydman DR. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- Calingasan NY, Uchida K, Gibson GE. Protein-bound acrolein: a novel marker of oxidative stress in Alzheimer’s disease. J Neurochem. 1999;72:751–756. doi: 10.1046/j.1471-4159.1999.0720751.x. [DOI] [PubMed] [Google Scholar]
- Carmella SG, Chen M, Zhang Y, Zhang S, Hatsukami DK, Hecht SS. Quantitation of acrolein-derived (3-hydroxypropyl)mercapturic acid in human urine by liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry: effects of cigarette smoking. Chem Res Toxicol. 2007;20:986–990. doi: 10.1021/tx700075y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano R, Vire B, Pion M, Quivy V, Olive D, Hirsch I, Van Lint C, Collette Y. Active transcription of the human FASL/CD95L/TNFSF6 promoter region in T lymphocytes involves chromatin remodeling: role of DNA methylation and protein acetylation suggest distinct mechanisms of transcriptional repression. J Biol Chem. 2006;281:14719–14728. doi: 10.1074/jbc.M602373200. [DOI] [PubMed] [Google Scholar]
- Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S, Lee A, Al-Abed Y, Vlassara H, Bucala R, Cerami A. Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci U S A. 1997;94:13915–13920. doi: 10.1073/pnas.94.25.13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chariot P, Drogou I, de Lacroix-Szmania I, Eliezer-Vanerot MC, Chazaud B, Lombes A, Schaeffer A, Zafrani ES. Zidovudine-induced mitochondrial disorder with massive liver steatosis, myopathy, lactic acidosis, and mitochondrial DNA depletion. J Hepatol. 1999;30:156–160. doi: 10.1016/s0168-8278(99)80020-8. [DOI] [PubMed] [Google Scholar]
- Crump NT, Hazzalin CA, Bowers EM, Alani RM, Cole PA, Mahadevan LC. Dynamic acetylation of all lysine-4 trimethylated histone H3 is evolutionarily conserved and mediated by p300/CBP. Proc Natl Acad Sci U S A. 2011;108:7814–7819. doi: 10.1073/pnas.1100099108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Asuncion JG, del Olmo ML, Sastre J, Pallardo FV, Vina J. Zidovudine (AZT) causes an oxidation of mitochondrial DNA in mouse liver. Hepatology. 1999;29:985–987. doi: 10.1002/hep.510290353. [DOI] [PubMed] [Google Scholar]
- Desai VG, Lee T, Moland CL, Branham WS, Mittelstaedt RA, Lewis SM, Leakey JE, Fuscoe JC. Evaluation of Hepatic Mitochondria and Hematological Parameters in Zidovudine-Treated B6C3F(1) Mice. AIDS Res Treat. 2012;2012:317695. doi: 10.1155/2012/317695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserich JP, van der Vliet A, Handelman GJ, Halliwell B, Cross CE. Dietary antioxidants and cigarette smoke-induced biomolecular damage: a complex interaction. Am J Clin Nutr. 1995;62:1490S–1500S. doi: 10.1093/ajcn/62.6.1490S. [DOI] [PubMed] [Google Scholar]
- Fang JL, Beland FA. Long-term exposure to zidovudine delays cell cycle progression, induces apoptosis, and decreases telomerase activity in human hepatocytes. Toxicol Sci. 2009;111:120–130. doi: 10.1093/toxsci/kfp136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang JL, Han T, Wu Q, Beland FA, Chang CW, Guo L, Fuscoe JC. Differential gene expression in human hepatocyte cell lines exposed to the antiretroviral agent zidovudine. Arch Toxicol. 2014;88:609–623. doi: 10.1007/s00204-013-1169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- Freiman JP, Helfert KE, Hamrell MR, Stein DS. Hepatomegaly with severe steatosis in HIV-seropositive patients. AIDS. 1993;7:379–385. doi: 10.1097/00002030-199303000-00012. [DOI] [PubMed] [Google Scholar]
- Galle PR, Hofmann WJ, Walczak H, Schaller H, Otto G, Stremmel W, Krammer PH, Runkel L. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995;182:1223–1230. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, Longshore D, Morton SC, Orlando M, Shapiro M. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol. 2002;63:179–186. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- Ghare S, Patil M, Hote P, Suttles J, McClain C, Barve S, Joshi-Barve S. Ethanol inhibits lipid raft-mediated TCR signaling and IL-2 expression: potential mechanism of alcohol-induced immune suppression. Alcohol Clin Exp Res. 2011;35:1435–1444. doi: 10.1111/j.1530-0277.2011.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghare SS, Joshi-Barve S, Moghe A, Patil M, Barker DF, Gobejishvili L, Brock GN, Cave M, McClain CJ, Barve SS. Coordinated histone H3 methylation and acetylation regulate physiologic and pathologic fas ligand gene expression in human CD4+ T cells. J Immunol. 2014;193:412–421. doi: 10.4049/jimmunol.1400055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobejishvili L, Avila DV, Barker DF, Ghare S, Henderson D, Brock GN, Kirpich IA, Joshi-Barve S, Mokshagundam SP, McClain CJ, Barve S. S-adenosylmethionine decreases lipopolysaccharide-induced phosphodiesterase 4B2 and attenuates tumor necrosis factor expression via cAMP/protein kinase A pathway. J Pharmacol Exp Ther. 2011;337:433–443. doi: 10.1124/jpet.110.174268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- Holtz-Heppelmann CJ, Algeciras A, Badley AD, Paya CV. Transcriptional regulation of the human FasL promoter-enhancer region. J Biol Chem. 1998;273:4416–4423. doi: 10.1074/jbc.273.8.4416. [DOI] [PubMed] [Google Scholar]
- Hristova M, Spiess PC, Kasahara DI, Randall MJ, Deng B, van der Vliet A. The tobacco smoke component, acrolein, suppresses innate macrophage responses by direct alkylation of c-Jun N-terminal kinase. Am J Respir Cell Mol Biol. 2012;46:23–33. doi: 10.1165/rcmb.2011-0134OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AA, Ray AS, Hanes J, Suo Z, Colacino JM, Anderson KS, Johnson KA. Toxicity of antiviral nucleoside analogs and the human mitochondrial DNA polymerase. J Biol Chem. 2001;276:40847–40857. doi: 10.1074/jbc.M106743200. [DOI] [PubMed] [Google Scholar]
- Kalyesubula R, Kagimu M, Opio KC, Kiguba R, Semitala CF, Schlech WF, Katabira ET. Hepatotoxicity from first line antiretroviral therapy: an experience from a resource limited setting. Afr Health Sci. 2011;11:16–23. [PMC free article] [PubMed] [Google Scholar]
- Kanzler S, Galle PR. Apoptosis and the liver. Semin Cancer Biol. 2000;10:173–184. doi: 10.1006/scbi.2000.0318. [DOI] [PubMed] [Google Scholar]
- Kavurma MM, Khachigian LM. Signaling and transcriptional control of Fas ligand gene expression. Cell Death Differ. 2003;10:36–44. doi: 10.1038/sj.cdd.4401179. [DOI] [PubMed] [Google Scholar]
- Kuhla A, Thrum M, Schaeper U, Fehring V, Schulze-Topphoff U, Abshagen K, Vollmar B. Liver-specific Fas silencing prevents galactosamine/lipopolysaccharide-induced liver injury. Apoptosis. 2015;20:500–511. doi: 10.1007/s10495-015-1088-2. [DOI] [PubMed] [Google Scholar]
- Li-Weber M, Krammer PH. Function and regulation of the CD95 (APO-1/Fas) ligand in the immune system. Semin Immunol. 2003;15:145–157. doi: 10.1016/s1044-5323(03)00030-7. [DOI] [PubMed] [Google Scholar]
- Liang G, Lin JC, Wei V, Yoo C, Cheng JC, Nguyen CT, Weisenberger DJ, Egger G, Takai D, Gonzales FA, Jones PA. Distinct localization of histone H3 acetylation and H3-K4 methylation to the transcription start sites in the human genome. Proc Natl Acad Sci U S A. 2004;101:7357–7362. doi: 10.1073/pnas.0401866101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Snyder P, Borgens RB, Shi R. Hydralazine rescues PC12 cells from acrolein-mediated death. J Neurosci Res. 2006;84:219–227. doi: 10.1002/jnr.20862. [DOI] [PubMed] [Google Scholar]
- Lloyd-Richardson EE, Stanton CA, Papandonatos GD, Betancourt RM, Stein M, Tashima K, Morrow K, Niaura R. HIV-positive smokers considering quitting: differences by race/ethnicity. Am J Health Behav. 2008;32:3–15. doi: 10.5555/ajhb.2008.32.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell MA, Markesbery WR. Ratio of 8-hydroxyguanine in intact DNA to free 8-hydroxyguanine is increased in Alzheimer disease ventricular cerebrospinal fluid. Arch Neurol. 2001;58:392–396. doi: 10.1001/archneur.58.3.392. [DOI] [PubMed] [Google Scholar]
- Malhi H, Guicciardi ME, Gores GJ. Hepatocyte death: a clear and present danger. Physiol Rev. 2010;90:1165–1194. doi: 10.1152/physrev.00061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan JP, Shah SS. Prevention of perinatal HIV transmission during pregnancy. J Antimicrob Chemother. 2000;46:657–668. doi: 10.1093/jac/46.5.657. [DOI] [PubMed] [Google Scholar]
- Miguez MJ, Shor-Posner G, Morales G, Rodriguez A, Burbano X. HIV treatment in drug abusers: impact of alcohol use. Addict Biol. 2003;8:33–37. doi: 10.1080/1355621031000069855. [DOI] [PubMed] [Google Scholar]
- Moghe A, Ghare S, Lamoreau B, Mohammad M, Barve S, McClain C, Joshi-Barve S. Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol Sci. 2015;143:242–255. doi: 10.1093/toxsci/kfu233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad MK, Avila D, Zhang J, Barve S, Arteel G, McClain C, Joshi-Barve S. Acrolein cytotoxicity in hepatocytes involves endoplasmic reticulum stress, mitochondrial dysfunction and oxidative stress. Toxicol Appl Pharmacol. 2012;265:73–82. doi: 10.1016/j.taap.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Strand S, Hug H, Heinemann EM, Walczak H, Hofmann WJ, Stremmel W, Krammer PH, Galle PR. Drug-induced apoptosis in hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand system and involves activation of wild-type p53. J Clin Invest. 1997;99:403–413. doi: 10.1172/JCI119174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat JE, Harris RE, Haley NJ, Wynder EL. Cigarette smoking and plasma cholesterol. Am Heart J. 1991;121:141–147. doi: 10.1016/0002-8703(91)90967-m. [DOI] [PubMed] [Google Scholar]
- Nunez M, Soriano V. Hepatotoxicity of antiretrovirals: incidence, mechanisms and management. Drug Saf. 2005;28:53–66. doi: 10.2165/00002018-200528010-00004. [DOI] [PubMed] [Google Scholar]
- Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD, Investigators HIVOS. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- Papadopoulou MV, Bloomer WD. DNA repair mechanisms are involved in the hypoxia-dependent toxicity of NLCQ-1 (NSC 709257) and its synergistic interaction with alkylating agents. In Vivo. 2007;21:175–180. [PubMed] [Google Scholar]
- Pianko S, Patella S, Ostapowicz G, Desmond P, Sievert W. Fas-mediated hepatocyte apoptosis is increased by hepatitis C virus infection and alcohol consumption, and may be associated with hepatic fibrosis: mechanisms of liver cell injury in chronic hepatitis C virus infection. J Viral Hepat. 2001;8:406–413. doi: 10.1046/j.1365-2893.2001.00316.x. [DOI] [PubMed] [Google Scholar]
- Price JC, Thio CL. Liver disease in the HIV-infected individual. Clin Gastroenterol Hepatol. 2010;8:1002–1012. doi: 10.1016/j.cgh.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purevjav E, Nelson DP, Varela JJ, Jimenez S, Kearney DL, Sanchez XV, DeFreitas G, Carabello B, Taylor MD, Vatta M, Shearer WT, Towbin JA, Bowles NE. Myocardial Fas ligand expression increases susceptibility to AZT-induced cardiomyopathy. Cardiovasc Toxicol. 2007;7:255–263. doi: 10.1007/s12012-007-9004-9. [DOI] [PubMed] [Google Scholar]
- Roh TY, Cuddapah S, Cui K, Zhao K. The genomic landscape of histone modifications in human T cells. Proc Natl Acad Sci U S A. 2006;103:15782–15787. doi: 10.1073/pnas.0607617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh TY, Cuddapah S, Zhao K. Active chromatin domains are defined by acetylation islands revealed by genome-wide mapping. Genes Dev. 2005;19:542–552. doi: 10.1101/gad.1272505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, Haughey N, Cutler R, Tamara A, Turchan J, Pardo C, Vargas D, Nath A. Novel markers of oxidative stress in actively progressive HIV dementia. J Neuroimmunol. 2004;157:176–184. doi: 10.1016/j.jneuroim.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Sakata K, Kashiwagi K, Sharmin S, Ueda S, Irie Y, Murotani N, Igarashi K. Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochem Biophys Res Commun. 2003;305:143–149. doi: 10.1016/s0006-291x(03)00716-2. [DOI] [PubMed] [Google Scholar]
- Satoh K, Yamada S, Koike Y, Igarashi Y, Toyokuni S, Kumano T, Takahata T, Hayakari M, Tsuchida S, Uchida K. A 1-hour enzyme-linked immunosorbent assay for quantitation of acrolein- and hydroxynonenal-modified proteins by epitope-bound casein matrix method. Anal Biochem. 1999;270:323–328. doi: 10.1006/abio.1999.4073. [DOI] [PubMed] [Google Scholar]
- Schattenberg JM, Worns MA, Zimmermann T, He YW, Galle PR, Schuchmann M. The role of death effector domain-containing proteins in acute oxidative cell injury in hepatocytes. Free Radic Biol Med. 2012;52:1911–1917. doi: 10.1016/j.freeradbiomed.2012.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senise JF, Castelo A, Martinez M. Current treatment strategies, complications and considerations for the use of HIV antiretroviral therapy during pregnancy. AIDS Rev. 2011;13:198–213. [PubMed] [Google Scholar]
- Sturt AS, Dokubo EK, Sint TT. Antiretroviral therapy (ART) for treating HIV infection in ART-eligible pregnant women. Cochrane Database Syst Rev. 2010:CD008440. doi: 10.1002/14651858.CD008440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Ito S, Nishio N, Tanaka Y, Chen N, Isobe K. Acrolein induced both pulmonary inflammation and the death of lung epithelial cells. Toxicol Lett. 2014;229:384–392. doi: 10.1016/j.toxlet.2014.06.021. [DOI] [PubMed] [Google Scholar]
- Szabados E, Fischer GM, Toth K, Csete B, Nemeti B, Trombitas K, Habon T, Endrei D, Sumegi B. Role of reactive oxygen species and poly-ADP-ribose polymerase in the development of AZT-induced cardiomyopathy in rat. Free Radic Biol Med. 1999;26:309–317. doi: 10.1016/s0891-5849(98)00199-3. [DOI] [PubMed] [Google Scholar]
- Teto G, Kanmogne GD, Torimiro JN, Alemnji G, Nguemaim FN, Takou D, Nanfack A, Tazoacha A. Lipid peroxidation and total cholesterol in HAART-naive patients infected with circulating recombinant forms of human immunodeficiency virus type-1 in Cameroon. PLoS One. 2013;8:e65126. doi: 10.1371/journal.pone.0065126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara H, Haruta T, Todoroki Y, Hiraoka M, Noiri E, Maeda M, Mayumi M. Oxidant and antioxidant activities in childhood meningitis. Life Sci. 2002;71:2797–2806. doi: 10.1016/s0024-3205(02)02137-9. [DOI] [PubMed] [Google Scholar]
- Vassimon HS, Deminice R, Machado AA, Monteiro JP, Jordao AA. The association of lipodystrophy and oxidative stress biomarkers in HIV-infected men. Curr HIV Res. 2010;8:364–369. doi: 10.2174/157016210791330347. [DOI] [PubMed] [Google Scholar]
- website, A., 2016. Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States.
- Woreta TA, Sutcliffe CG, Mehta SH, Brown TT, Higgins Y, Thomas DL, Torbenson MS, Moore RD, Sulkowski MS. Incidence and risk factors for steatosis progression in adults coinfected with HIV and hepatitis C virus. Gastroenterology. 2011;140:809–817. doi: 10.1053/j.gastro.2010.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Chen L, Jin H, Shao J, Wu L, Lu Y, Zheng S. Activation of Fas death receptor pathway and Bid in hepatocytes is involved in saikosaponin D induction of hepatotoxicity. Environ Toxicol Pharmacol. 2015;41:8–13. doi: 10.1016/j.etap.2015.11.005. [DOI] [PubMed] [Google Scholar]