Figure 5. The hNaa60-specific β3–β4 loop.
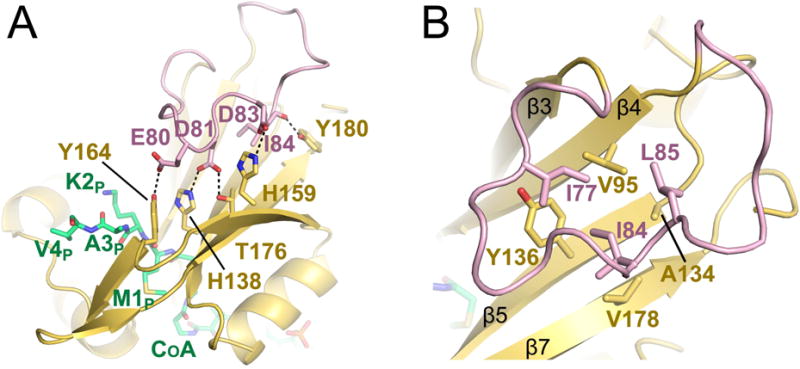
A) Residues D81 and D83 (pink sticks) of the extended β3–β4 loop of hNaa60 (shown in pink) that form salt bridges with residues H138 and H159 (yellow sticks) of β5 and β7 are shown. E80 and D81 (pink sticks) that form hydrogen bonds with Y164 and T176 (yellow sticks) of β6 and the carbonyl backbone of I84 (pink sticks) that form a hydrogen bond with Y180 (yellow sticks) are also shown. The CoA-Ac-MKAV is shown in green sticks. B) Residues I77, I84 and L85 (pink sticks) that form a hydrophobic interior of the loop and participate in van der Waals interactions with residues V95, A134, Y136 and V178 (yellow sticks) of β5, β6 and β7 strands is shown. See also Figure S4.
