Fig. 5.
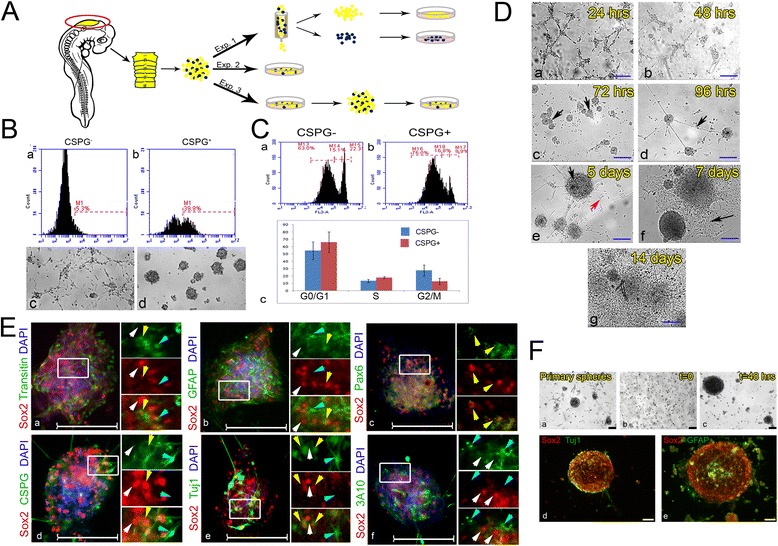
Formation of neurospheres with self-renewal and differentiation capacities from hindbrain-derived Sox2+ cells. A Scheme of experiments using primary cultures prepared from 18HH chick hindbrains. B Magnetic column-based separation of CSPG+ HB cells. Enrichment of CSPG+ compared to CSPG– samples assayed by flow cytometry (a,b). Culture of CSPG– and CSPG+ cells, respectively, shown in bright field 96 h post-separation (80 hindbrains used to obtain cell suspension) (c,d). C Cell cycle analysis by flow-cytometry on column-based separation of CSPG+ vs. CSPG– cells that were stained with propidium iodide. CSPG– and CSPG+ fractions, respectively (a,b). Graphic representation of cell cycle analysis results (c). Analysis was repeated twice, each time with three technical repeats (P value for each replica > 0.05, t test). D Bright-field views of primary cultures of hindbrain cells documented after plating from 24 h to 14 days (a–g). Arrows in (c) indicate newly formed spheres; in (d) an axon connecting two spheres; in (e) an intact sphere (black) with adjacent morphologically differentiating cell (red); and in (f) morphologically differentiated cells generated by collapsed sphere. E Co-staining of hindbrain-originated spheres (10 hindbrains used for each primary culture) for Sox2 with Transitin (a), GFAP (b), Pax6 (c), CSPG (d), Tuj1 (e), and 3A10 (f). High magnification views of the boxed areas are shown to the right of each panel in different channels. White, yellow or blue arrowheads indicate cells stained for Sox2 alone, for both markers or for the relevant marker alone, respectively. F Secondary sphere formation. Primary spheres (a) were dissociated and re-plated as single cells (b). Secondary spheres appeared after 48 h (c). Spheres were co-stained for Sox2 with Tuj1 (d) or GFAP (e). Scale bars in D,E = 100 μm, in F = 75 μm (43 hindbrains used to obtain primary spheres)
