Fig. 1.
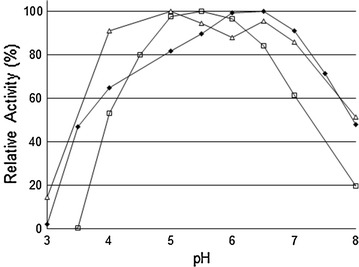
pH-dependent relative activity of THSAbf (n = 4). Enzyme activity was measured by monitoring the release of pNP or reducing sugars at different pH values. Various buffer systems were utilized: citrate (pH 3–6), phosphate (pH 6–8), and citrate–phosphate (pH 5.5–6.5). Different substrates were tested. Filled diamonds pNP-Araf; open squares pNP-Xylp; open triangles LWAX. pH profiles for pNP-Araf, pNP- Xylp, and LWAX. 100 % activity was taken as the activity at the optimal pH for a given substrate (relative activity)
