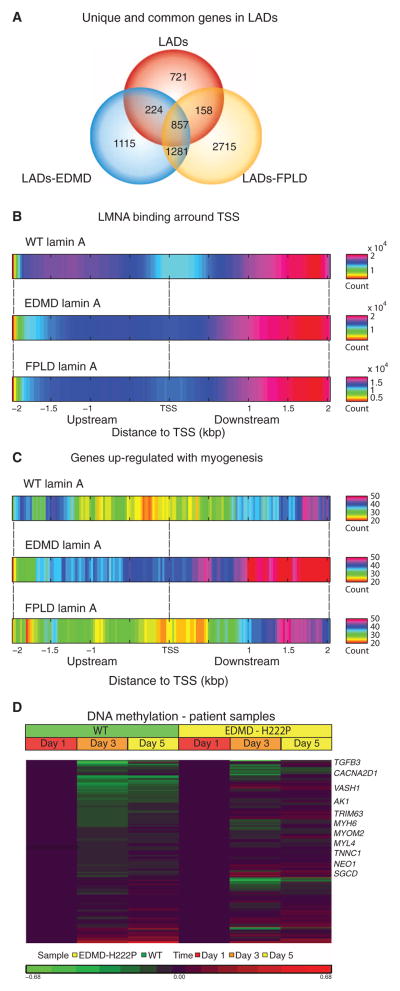
Fig. 1. Promiscuous formation of short LADs by mutation-bearing lamin A/C proteins is associated with allele-specific perturbation of myogenic programs.
Interactions between lamin A nuclear envelope protein and chromatin domains were assessed by Dam–lamin A fusion protein using DamID-seq [wild-type (WT), p.R453W EDMD, and p.R482W FPLD] in differentiating human myoblasts. LADs were scored and compared between the three lamin A/C variants. (A) Overlap in LADs (and genes found in LADs) among the three variants. Mutant lamin A proteins showed promiscuous formation of new LADs that were of shorter length compared to WT (fig. S1). (B) WT lamin A showed loss of LADs at TSS, whereas mutant lamin A showed abnormal increased association with start sites. WT and mutations in lamin A showed most binding downstream of TSS. (C) Restriction of analysis to transcript units associated with myogenic differentiation showed broad loss of lamin A binding with WT lamin A, consistent with euchromatinization of myogenic loci at the myotube stage. Lamin A containing an EDMD mutation (p.R453W) showed persistent heterochromatinization of myogenic loci, whereas p.R482W FPLD mutation retained appropriate loss of LADs similar to WT. (D) DNA methylation status of myogenesis-associated loci (n = 322) in fibroblasts from EDMD patients [carrying the LMNA mutation (p.H222P)] transfected with an MyoD expression vector shows expected loss in methylation similar to cells from control subjects. Genes involved in skeletal and cardiac muscle development that showed persistent methylation are marked at the left.