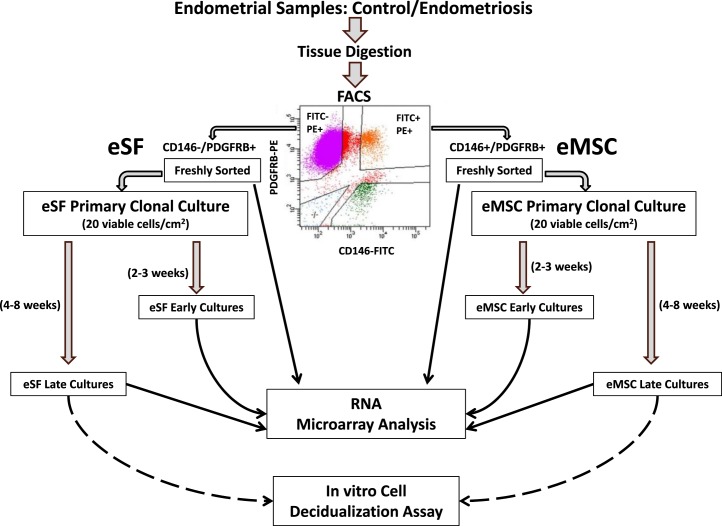
FIG. 1.
Experimental design. Pure populations of endometrial mesenchymal stem cells (eMSC) and endometrial stromal fibroblasts (eSF) were isolated by fluorescence-activated cell sorting (FACS) after enzymatic digestion of endometrial tissue. The four-color FACS excluded leukocytes and epithelial cells, sorting the remaining cells according to the binding of fluorescein isothiocyanate (FITC)-labeled antibody to cluster of differentiation 146 (CD146) and of phycoerythrin (PE)-labeled antibody to platelet-derived growth factor receptor beta (PDGFRB), isolating two populations: FITC+/PE+ eMSC co-expressing CD146 and PDGFR, and FITC−/PE+ eSF that express PDGFRB but not CD146. An aliquot of each freshly sorted population was processed for RNA extraction and microarrray analysis. When FACS yields were sufficient, the remainder of the sorted eMSC and eSF were established in primary culture at clonal density (10–20 viable cells/cm2). Replicates of primary eMSC and eSF clonal cultures were harvested at early (2–3 wk) and late (4–8 wk) stages of colony development and processed for RNA extraction and microarrray analysis. Cells harvested from late cultures were also used for in vitro decidualization assays.