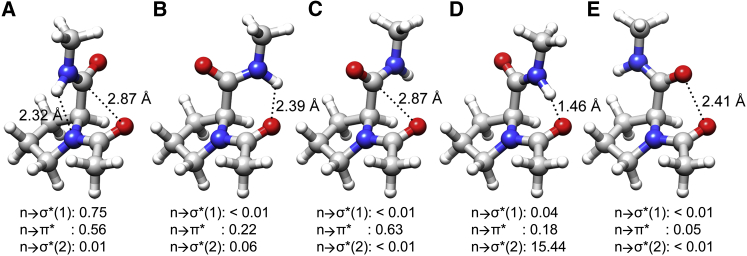
Figure 4.
Molecular plots of Ace-Pro-NMe, representing different regions of the potential energy profile and corresponding to the points labeled as (A)–(E) in Fig. 3. (A–E) The ψ values assumed for these structures are −22.9°, 102.1°, 137.1°, 42.1°, and −122.9°, respectively. To begin with, the hydrogen atoms were optimized in (A) as described in Materials and Methods. Conformation (A) is stabilized by both Ni+1-Hi+1…Ni H-bond (n → σ∗ (1)) and n → π∗ interactions, whereas (B) and (C) are stabilized by Oi-1…Hi+1-Ni+1 H-bond (n → σ∗ (2)) and n → π∗ interactions, respectively. Conformation (A) corresponds to the experimental structure and is energetically the most stable conformation, whereas the energy difference between (B) and (C) is only ∼0.17 kcal/mol. Although (D) is stabilized by the Oi-1…Hi+1-Ni+1 H-bond, the Hi+1 and Oi-1 are too close (1.46 Å, far less than the sum of van der Waals radii (2.72 Å)), and as a result there is a serious steric clash between these atoms. In (E), the two carbonyl oxygen atoms carrying partial negative charges are repulsed by the close approach of these atoms. The second-order perturbation energies of all three interactions are provided for each conformation. To see this figure in color, go online.