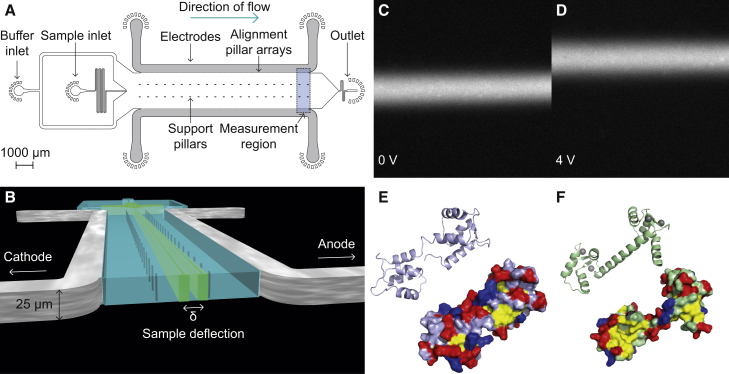
Figure 1.
Experimental design. (A) The design of the microfluidic free-flow electrophoresis device used in this study. Integrated metal electrodes are shown in gray. Inlets for the sample and buffer are indicated. Support pillars for the channel ceiling and the pillar array utilized for the electrode integration and alignment are also shown. The sample deflection is measured at the end of the electrophoresis channel (blue shaded area). (B) A pseudo-three-dimensional representation of the device showing a cross section of the main electrophoresis channel. Upon application of an electric potential, the sample is deflected from its path at the center of the channel. The scale bar refers to the vertical dimension. (C) Fluorescence image of 1 μM calmodulin in the buffer containing EDTA at 0 V. (D) Fluorescence image of 1 μM calmodulin in the buffer containing EDTA at an applied potential of 4 V. The negatively charged protein is deflected toward the anode. (E) The structure of calmodulin in the absence of Ca2+ shown as a ribbon diagram (top) and as a surface plot with hydrophobic residues (isoleucine, leucine, phenylalanine, and methionine) in yellow, acidic residues in red, and basic residues in blue (PDB: 1CFD) (22). (F) The structure of calmodulin with four Ca2+ (gray spheres), chelated by the four EF-hands, can be seen in the ribbon diagram (top), whereas the surface representation (bottom) reveals the exposure of two hydrophobic patches upon Ca2+ binding (PDB: 3CLN) (23).