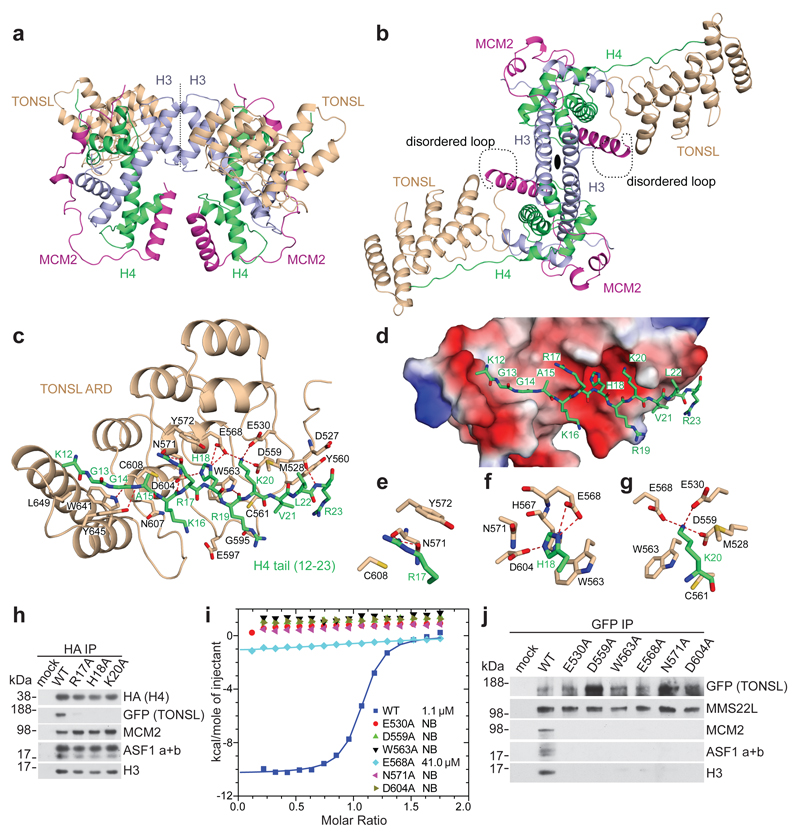
Figure 1. TONSL ARD interacts with the histone H4 tail.
a, b, Two different representative views of the overall structure of the TONSL ARD–MCM2 HBD–H3–H4 tetramer complex. c, Intermolecular interactions between TONSL ARD and the H4 tail. d, The electrostatic potential surface of ARD showing the acidic concave surface binding site for the H4 tail. e-g, Highlights of the inter-molecular interactions of H4 Arg17, His18 and Lys20 with ARD. h, Immunoprecipitation of soluble HA-SNAP-H4 wild type (WT) or mutant transfected into GFP-TONSL U-2-OS cells. i, ITC of TONSL ARD WT and mutants with H4 tail peptide. j, Immunoprecipitation of soluble GFP-TONSL WT or mutant. Data are representative of 3 independent experiments (h, j). Protein inputs, Extended Data Fig. 9b, c. Gel source data, Supplementary Fig. 1.