Figure 1.
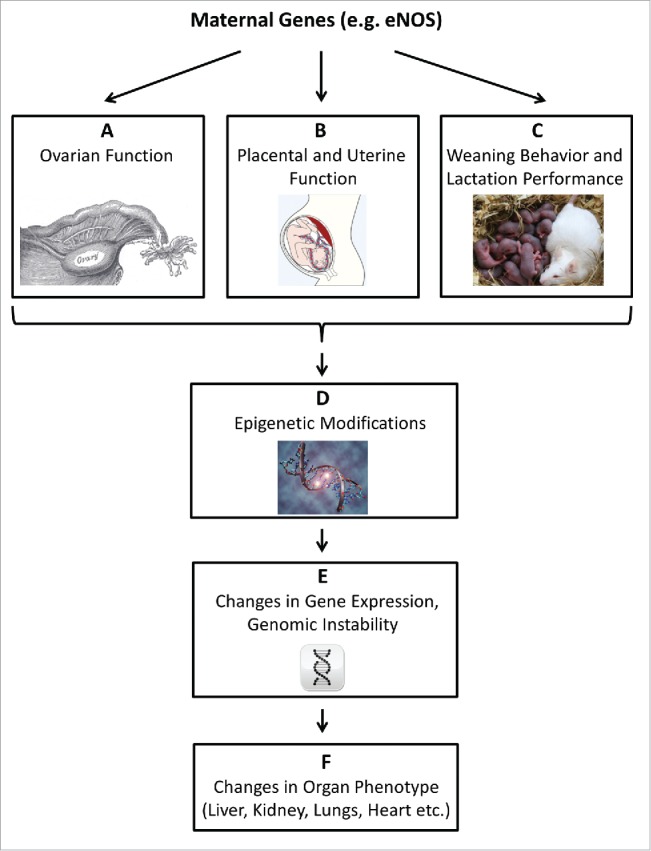
The advanced fetal programming hypothesis. The ‘fetal origin’ hypothesis proposes that adulthood cardiovascular, metabolic, and mental diseases originate through adaptation of the fetus to environmental conditions in early life. We proposed that maternal genetic defects might impact on the offspring phenotype via genomic-epigenomic interactions, without transmittance of the defective gene. Such interactions, here exemplified by the eNOS gene, could be mediated during 3 phases of reproduction (A-C). (A) Maternal gene dysfunction can alter ovary function: eNOS mediates physiological ovarian functions, such as blood flow and angiogenesis and is involved in oocyte meiotic maturation.49,50 (B) Maternal gene dysfunction can alter placental and uterine function: eNOS plays a pivotal role in the control of placental function, and eNOS deficiency is associated with an unfavorable intrauterine environment.16,17 (C) Maternal gene dysfunction may alter weaning behavior and lactation performance: eNOS is involved in behavioral processes and elicits regulatory functions in lactation.51,52 Alterations in maternal eNOS function thus may affect the offspring in the early postnatal phase.51,52 (D) The impact of maternal gene dysfunction on embryonal/fetal/neonatal environmental factors listed in A-C may trigger stable, long lasting epigenetic adaptation in the offspring.53-55 Epigenetic mechanisms encompass DNA methylation, non-coding RNAs, and chromatin modifications.56 (E) Epigenetic modification, specifically DNA methylation and non-coding RNAs, can result in permanently altered gene expression in the offspring.34 On a global scale, both DNA hypermethylation and DNA hypomethylation may cause genomic instability.57,58 (F) Altered gene expression and genomic instability triggered by epigenetic maladaptation can impact on the offspring phenotype by permanently altering organ structure and function.59,60
