Abstract
Consumption of red wine is associated with a decreased risk of several cardiovascular diseases (e.g., coronary artery disease, stroke), but unfortunately literature reports regarding ethanol’s effects on hemorheological parameters are not concordant. In the present study, red blood cell (RBC) deformability was tested via laser ektacytometry (LORCA, 0.3 – 30 Pa) using two approaches: 1) addition of ethanol to whole blood at 0.25% – 2% followed by incubation and testing in ethanol-free LORCA medium; 2) addition of ethanol to the LORCA medium at 0.25% – 6% then testing untreated native RBC in these media. The effects of ethanol on deformability for oxidatively stressed RBC were investigated as were changes of RBC aggregation (Myrenne Aggregometer) for cells in autologous plasma or 3% 70 kDa dextran.
Significant dose-related increases of RBC deformability were observed at 0.25% (p<0.05) and higher concentrations only if ethanol was in the LORCA medium; no changes occurred for cells previously incubated with ethanol then tested in ethanol-free medium. The impaired deformability of cells pre-exposed to oxidative stress was improved only if ethanol was in the LORCA medium. RBC aggregation decreased with concentration at 0.25% and higher for cells in both autologous plasma and dextran 70. Our results indicate that ethanol reversibly improves erythrocyte deformability and irreversibly decreases erythrocyte aggregation; the relevance of these results to the health benefits of moderate wine consumption require further investigation.
Keywords: alcohol, erythrocyte aggregation, erythrocyte deformability, oxidative stress
Introduction
Several epidemiological studies have shown that regular but moderate red wine consumption (i.e., not more than 10–20 g alcohol per day) results in a decreased risk for various cardiovascular diseases including coronary heart disease [8] [24], heart failure [18], intermittent claudication [19] and stroke [48]. This phenomenon (i.e., beneficial effects of moderate alcohol consumption) has been termed the “French Paradox” [45].
Studies of red wine consumption have confirmed that, in addition to red wine’s antioxidant phenolic components [16], ethanol plays a role in its beneficial cardiovascular effects. Ethanol favorably modifies hemostasis leading to reduced levels of certain coagulation factors (e.g., fibrinogen, factor VII and von Willebrand factor) and of platelet function [46] [49]; enhanced fibrinolysis due to elevated levels of tissue-type plasminogen activator has also been observed [2] [49]. Alterations of plasma lipid profiles with an increase in high-density lipoprotein and a decrease in low density lipoprotein cholesterol concentrations have been reported [23] [27], and ethanol enhances the production of the vasodilator endothelial nitric-oxide [1].
The Framingham Study, as well as other literature reports, have clearly shown that hemorheological parameters can be considered as potential cardiovascular risk factors, and that abnormalities of these parameters contribute to the development of cardiovascular diseases [9] [31] [34]. Several in vivo and in vitro experiments have evaluated the effects of ethanol on different hemorheological factors but the results are not in complete agreement. While in vitro studies exploring the effects of ethanol addition to blood indicate no changes of hematocrit or whole blood viscosity [22] [26] [37], alcohol consumption leads to dehydration without increased hematocrit [22] [37] but with an elevation of whole blood and plasma viscosity [20] [26] [41] [51]. Regular but modest ethanol consumption is associated with a decreased level of plasma fibrinogen [17] [49] which was correlated with a reduction of plasma viscosity [29]. In contrast, a recent study has reported no changes of fibrinogen, hematocrit or blood viscosity after moderate vodka consumption for two weeks [32].
The in vitro effects of red wine and of alcohol-free red wine (AFRW) on red blood cell (RBC) aggregation and deformability have recently been reported: 1) both red wine and AFRW inhibited aggregation in a dose-dependent manner, with effects seen beginning at a 0.1% concentration; 2) red wine tended to have a greater effect compared to AFRW; 3) neither red wine nor AFRW affected RBC deformability although AFRW did provide partial protection from oxidative damage [44].
Given the current uncertainty regarding the specific hemorheological consequences of alcohol, the present in vitro study was designed to further explore possible effects of pure ethanol on RBC deformability and aggregation.
Methods
Blood and RBC suspensions
Venous blood samples were obtained by sterile venipuncture from healthy adult laboratory personnel and anticoagulated with EDTA (1.5 mg/ml). Blood donors did not consume any ethanol-containing products within 24 hours of sampling. The study was approved by the Human IRB, University of Southern California, Los Angeles, CA.
Two general approaches were utilized to evaluate ethanol effects on RBC deformability: 1) direct addition of ethanol to whole blood followed by incubation and deformability testing; 2) addition of ethanol only to the viscous suspending medium used for RBC deformability measurements. All studies used reagent grade ethanol (Sigma-Aldrich Co., St. Louis, MO, USA) and isotonic phosphate buffered saline (PBS, 290 mOsm/kg, pH = 7.4) was used as a dilution control. In the direct addition studies, ethanol was added to whole blood or a RBC-plasma suspension to achieve final concentrations of 0.25, 0.50, 1 and 2%, following which these samples were incubated at room temperature for one hour then studied. In the other approach, designed to evaluate the more-immediate effects of ethanol, the alcohol was added directly to the viscous dextran medium used for deformability measurements (see below) at concentrations of 0.25, 0.50, 1, 2, 3, 4, 5 and 6%, following which untreated RBC were suspended in these media then measured.
Aggregation measurements used 40% hematocrit suspensions of RBC in autologous plasma or in a 70 kDa dextran solution (3% in PBS, Sigma). In these studies, RBC were initially suspended in plasma at a 40% hematocrit, alcohol added (0.25, 0.50, 1 and 2%), and incubated for one hour at room temperature. RBC-plasma samples were then tested without further processing, while RBC to be suspended in 3% 70 kDa dextran were washed twice with PBS then re-suspended in the dextran at 40% hematocrit.
To examine the in vitro effect of ethanol in the presence of oxidative stress, alcohol was added to whole blood (0.25, 0.50, 1 and 2%) together with the free radical generator phenazine methosulfate (PMS, Sigma) at a concentration of 500 μM. The ethanol-PMS samples were incubated at 37°C for 2 hours then tested in alcohol-free LORCA media. In one series, whole blood was treated only with PMS and alcohol added only to the viscous medium used for deformability measurements.
RBC deformability and aggregation
Erythrocyte deformation in response to defined shear forces was determined by ektacytometry (LORCA, Laser-assisted Optical Rotational Cell Analyzer; R&R Mechatronics, Hoorn, Netherlands). In this instrument, a dilute suspension of RBC (~ 2 × 107 RBC/ml) in a viscous medium is placed in the gap of a Couette shearing system having a laser-diode projected through the gap. The presence of RBC in the gap creates a diffraction pattern that changes from circular to elliptical as cells deform and elongate. The pattern is captured and analyzed by a video camera and computer system that provides an elongation index (EI) as the (length-width)/(length+width) of the pattern for each shear stress. For some results, EI-stress data were fitted to a Lineweaver-Burke type non-linear equation that yields the maximum EI at infinite shear stress (EImax) and the stress required to achieve one-half of this maximum value (SS1/2) [3] [33]. The ratio of (SS1/2) / (EImax) was used as an empirical approach to account for differing EImax levels. Data fitting and analysis were carried out using non-linear regression (GraphPad Prism, GraphPad Software, La Jolla, CA). In the present study, the viscous medium used in the LORCA was an isotonic solution of 70 kDa dextran (297 mOsm/kg, η=28.4 mPa·s at 37 °C); the shear stress was varied, in steps, from 0.3 to 30 Pa [28] [33].
Erythrocyte aggregation in plasma and in 3% 70 kDa dextran was determined with a Myrenne aggregometer (model MA-1, Myrenne GmbH, Roetgen, Germany) that employs measurement of infrared light transmission through a RBC suspension. RBC are initially disaggregated by high shear (600 s−1) following which shear is abruptly stopped or reduced to 3 s−1 and light transmission integrated for 10 s. The instrument provides two dimensionless indices of RBC aggregation (M, aggregation at stasis; M1, at very low shear); both M and M1 increase with enhanced aggregation [6] [52].
Miscellaneous
RBC shape was evaluated by DIC light microscopy (model BX50F; Olympus, Tokyo, Japan). Paired t-tests were used to test changes from control, with significance accepted at p<0.05.
Results
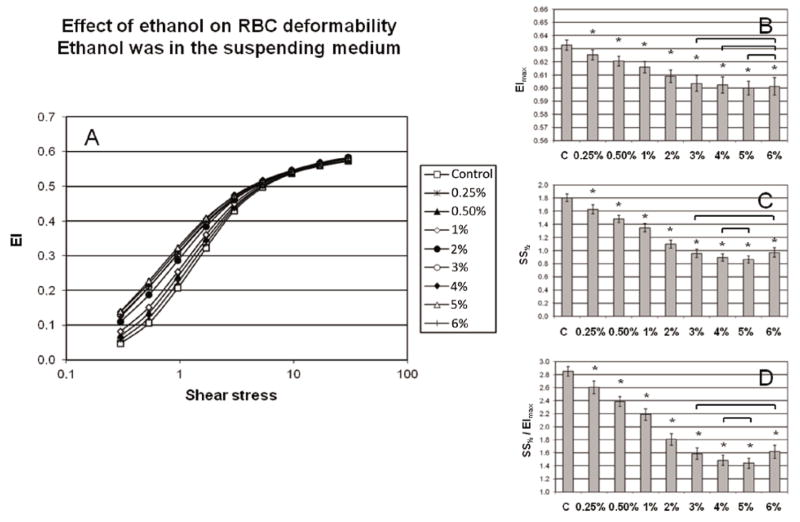
The effects of ethanol on RBC deformability depended on the manner in which cells were exposed to alcohol (see Methods): 1) addition to whole blood followed by a one-hour incubation caused no change in deformability if ethanol was not present in the LORCA media (Figure 1A); 2) addition to the LORCA media and testing of non-incubated cells resulted in significant, dose-dependent deformability increase (Figure 2A). Analyses obtained using the Lineweaver-Burke regression indicated that neither EImax, SS1/2 nor their ratio differed from control for incubated cells tested in alcohol-free media (Figure 1, panels B, C and D). Conversely, EImax, SS1/2 and their ratio for non-incubated cells decreased with alcohol concentration of the LORCA media (Figure 2, panels B, C and D). Initial changes of these three deformability parameters occurred at 0.25% (~ 11% decrease, p<0.05). Note that there was a bi-phasic effect with increasing concentration: both SS1/2 and the SS1/2/EImax ratio progressively decreased (i.e., increased deformability) with a plateau at 4–5% followed by an increase at 6%. Nevertheless, at the highest concentration (6%), SS1/2 and the SS1/2 / EImax ratio decreased by 46% (SS1/2) and 43% (ratio), indicating that the presence of ethanol in the LORCA media resulted in significant increases (p<0.05) of RBC deformability.
Figure 1.
A: RBC deformation as an elongation index (EI) versus shear stress when ethanol (0.25–2%) was added to whole blood, the suspension incubated, and the cells tested in ethanol-free LORCA media. B: EImax, C: SS½ and D: SS½ / EImax calculated using non-linear regression of the Lineweaver-Burke equation. Control means phosphate buffered saline treated samples. N=7, values are mean ± SD. No significant differences were detected.
Figure 2.
A: RBC deformation as an elongation index (EI) versus shear stress when ethanol (0.25–6%) was added to the suspending medium of the LORCA ektacytometer; RBC were not pre-incubated with ethanol. B: EImax, C: SS½ and D: SS½ / EImax calculated using non-linear regression of the Lineweaver-Burke equation. Control means only a phosphate buffered saline dilution added to the LORCA media. N=7, values are mean ± SD. Stars represent significant differences from control samples at p<0.05, while links show differences which are not significant.
The effects of ethanol on erythrocyte deformability when cells were oxidatively stressed by the free radical generator phenazine methosulfate were also studied using the LORCA ektacytometer. As expected [5], incubation with 500 μM PMS alone caused a significant decrease of deformability (Figures 3A and 4A). Ethanol-related changes of deformability again depended strongly upon the manner in which cells were exposed to alcohol. RBC incubated for 2 hours with ethanol plus PMS then tested in alcohol-free LORCA media exhibited significant decreases of deformability from PMS alone (Figure 3A). Under these conditions, EImax for PMS treated cells was unaffected by the presence of ethanol during incubation while both SS1/2 and the SS1/2 / EImax ratio increased starting at 0.5% and at 2% were ~20% above PMS-only RBC (Figure 3, panels B, C and D). However, PMS treated cells tested with alcohol in the LORCA media exhibited improvements of deformability compared to PMS alone (Figure 4A): the three parameters showed significant improvement beginning at 0.25% with SS1/2 and the SS1/2 / EImax ratio ~17% lower at 2% ethanol (Figure 4, panels B, C and D).
Figure 3.
A: RBC deformation as an elongation index (EI) versus shear stress for whole blood incubated with ethanol (0.25–2%) + 500 μM phenazine methosulfate (PMS); following incubation the cells were tested in ethanol-free LORCA media. B: EImax, C: SS½ and D: SS½ / EImax calculated using non-linear regression of the Lineweaver-Burke equation. Control means phosphate buffered saline treated samples. N=7, values are mean ± SD. Stars represent significant differences from PMS treated samples at p<0.05, while links show differences which are not significant.
Figure 4.
A: RBC deformation as an elongation index (EI) versus shear stress for whole blood incubated with 500 μM phenazine methosulfate (PMS); following incubation, cell deformability was measured in LORCA media containing 0.25–2% ethanol. B: EImax, C: SS½ and D: SS½ / EImax calculated using non-linear regression of the Lineweaver-Burke equation. Control means phosphate buffered saline treated samples. N=7, values are mean ± SD. Stars represent significant differences from PMS treated samples at p<0.05, while link shows a difference which is not significant.
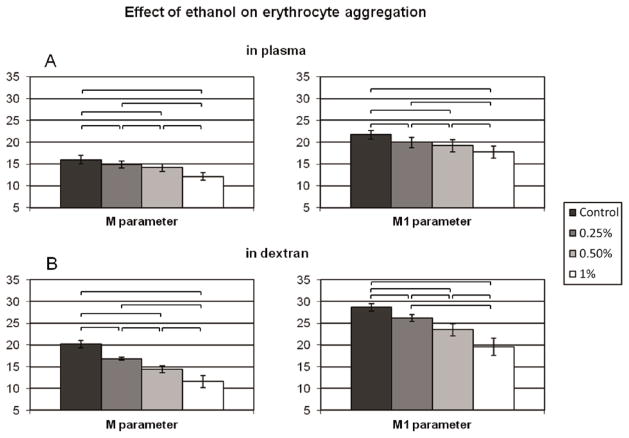
Erythrocyte aggregation in autologous plasma (Figure 5A) or in 3% 70 kDa dextran solution (Figure 5B) showed significant decreases (p<0.05) in a dose-dependent manner. The changes of the M and M1 indices were significant at 0.25% and above, with the greatest decreases at 1% alcohol: mean reductions of M and M1 parameters were 24% and 18% for aggregation in plasma and 43% and 32% for aggregation in dextran. At 2% ethanol the aggregometer was unable to detect RBC aggregate formation in either medium.
Figure 5.
Erythrocyte aggregation determined by Myrenne aggregometer. RBC were initially suspended in plasma at a 40% hematocrit, alcohol added (0, 0.25, 0.50 and 1%), incubated then tested without further processing, while RBC to be suspended in 3% 70 kDa dextran were washed twice with PBS then re-suspended in the dextran at 40% hematocrit. RBC aggregation A: in autologous plasma or B: in 3% 70 kDa dextran solution. No aggregation was measureable at 2% concentration (not shown). Control means phosphate buffered saline treated samples. N=7, values are mean ± SD. Links represent significant differences at p<0.05.
Morphological analysis using DIC light microscopy demonstrated that normal discocytes in PBS (Figure 6A) became somewhat echinocytic with 2% ethanol (Figure 6B). The viscous dextran medium used in the LORCA induces a slight stomatocytic transformation (Figure 6C) while erythrocytes retain their normal, discocytic shape in dextran with 2% alcohol (Figure 6D).
Figure 6.
Morphological appearance of erythrocytes visualized by DIC light microscopy. A: Untreated cells in PBS. B: Erythrocytes in PBS with 2% ethanol concentration. C: RBC in the LORCA viscous medium (dextran). D: Erythrocytes in dextran containing 2% ethanol.
Discussion
The direct relationship of our results to the French Paradox [45] is, at present, not certain; this paradox is associated with moderate yet frequent alcohol consumption whereas our findings reflect a one-time exposure to ethanol. Furthermore, it is important to note that the cardiovascular risk reduction associated with moderate red wine drinking is most likely related to the combined beneficial effects of red wine components (e.g., different polyphenols, ethanol): separate studies of these main components may not reflect the overall response seen with red wine. The majority of ethanol concentrations used herein greatly exceeds levels that are physiologically tolerable: in many locations throughout the world; intoxication and an inability to operate machinery (e.g., drive a car) are assumed at 0.08 to 0.1% ethanol. However, the results at 0.25% (i.e., improved deformability and decreased aggregation, Figures 2, 4 and 5) seem relevant to possible in vivo positive effects. Oonishi and Sakashita have reported improved filterability of human RBC at 0.2% and indicate that their results were obtained at “concentrations that are physiologically achievable in the blood” [43]. Furthermore, Mesquita et al. have shown that in vitro addition of ethanol at 0.31% decreases RBC aggregation, with this finding deemed of importance for “future changes in therapeutic approaches to situations such as alcoholic coma” [37]. It is therefore possible that moderate, routine consumption of ethanol (i.e., red wine) may have a cumulative effect that occurs at lower ethanol levels; such an effect was not evaluated as part of the current study.
Red blood cell deformability (i.e., the ability of the cell to adopt a new shape in response to deforming forces) is of both basic science and clinical interest: altered deformability can affect the rheological behavior of blood and, if markedly reduced, can impair in vivo tissue perfusion. There is general agreement regarding the factors affecting RBC deformability: cell shape and membrane surface area to volume ratio as “extrinsic” factors; membrane viscoelastic properties and cytosolic viscosity as “intrinsic” factors [13] [38]. Deviations from the normal resting biconcave shape, decreased area to volume ratio, higher membrane shear modulus and viscosity or elevated cytoplasmic viscosity tend to reduce deformability. The relative importance of each of these parameters for altering deformability can depend on the testing system and the level of applied forces; abnormal deformation behavior may be detected at low stress level forces but may not be evident when much higher forces are applied. In order to avoid assuming the appropriate stress or stresses for comparisons, we have elected to utilize a curve fitting approach over the entire range of shear stress (i.e., 0.3–30 Pa) in order to characterize RBC mechanical behavior by just two parameters (i.e., EImax and SS1/2); this approach has been validated and shown to be appropriate for various RBC populations [3] [33].
The results of our in vitro study clearly indicate that ethanol can improve RBC deformability when the cells are subjected to fluid stress in a defined shear field. These improvements were only observed when ethanol was in the viscous media used for ektacytometry testing (Figure 2) and were not present when cells were incubated with the alcohol but tested in alcohol-free viscous media (Figure 1). This need for sustained alcohol levels is supported by several in vivo experiments [15] [32] and in vitro studies suggesting that changes in the cell membrane are reversible [35] [53].
Comparing our deformability results to literature reports is somewhat problematic inasmuch as a variety of methods and ethanol exposure were used. For example, the effects of ethanol consumption depend on the drinking habits of the subjects tested. Using micropore filtration, studies have shown that RBC deformability is reduced in active alcoholics [7] [25]. On the other hand, ektacytometry results have shown increased deformability at high shear stresses 1.5 hours after the ethanol intake [15], while a recent study indicates no changes after moderate vodka consumption for two weeks [32]. Prior in vitro studies are also not in concordance: filterability measurements have demonstrated increased RBC deformability at physiological concentrations of ethanol [43], while a micropipette aspiration technique has shown that high, intolerable levels of ethanol decreases deformability [11]. Our results indicate a bi-phasic effect of ethanol: increasing improvement from 0.25% to 4–5%, while deformability at 6% was lower and similar to the 3% ethanol results (Figure 2).
It is interesting to speculate regarding the mechanisms responsible for the effects of ethanol on RBC deformability. As indicated above, four factors (e.g., morphology, geometry, membrane rheologic properties and cytoplasmic viscosity) can affect erythrocyte deformation behavior [13] [38]. Although ethanol can cause a discocyte-echinocyte shape change [35] [36], cells suspended in dextran + ethanol generally have a discoidal morphology (Figure 6). Ingested alcohol increases plasma osmolality [10] [47], thereby reducing cell volume [22], increasing surface to volume ratio and increasing cytoplasmic viscosity; the increased ratio favors deformability while the greater cytoplasmic viscosity has the opposite effect. Given that cells were always suspended in isotonic media, it thus seems most likely that ethanol affects the mechanical behavior of the membrane with its attached cytoskeleton. The importance of the cytoskeleton for the cell’s physical behavior has been shown in a detailed analysis of RBC membrane properties; the lipid bilayer cannot exhibit elasticity (i.e., shear modulus of elasticity) but merely flows when deformed [21].
Ethanol has a polar hydroxyl group soluble in aqueous media and hence must distribute within the exterior glycocalyx and the interior of the cell, while the non-polar part of the molecule is preferentially found in the lipid bilayer [14] [50]. The fluidity of the lipid portion can be altered by ethanol in a dose dependent manner: 1) up to 0.3% there is no change in the membrane’s external layer (TMA-DPH fluorescence) or its hydrophobic region (DPH fluorescence) [37]; 2) increased fluidity up to 1.6% as assayed by electron paramagnetic resonance [14]. Note, however, that a less viscous lipid bilayer is expected to only minimally affect overall cell deformability [21] [30] [38]. Thus the cytoskeleton, composed of spectrin, actin protein 4.1, band 4.2, ankyrin, adducin, dematin, tropomyosin, and tropomodulin, must be reversibly altered in a manner that decreases membrane shear modulus [39] [40]. In addition, it is possible that transmembrane proteins are involved [38], with the most likely molecular change a weakening of spectrin-actin linkages [21] [39] although cytoskeletal-transmembrane interactions may also occur [38].
The in vitro effect of ethanol in the presence of oxidative stress generated by phenazine methosulfate (PMS) was also examined (Figures 3 and 4). PMS is a well-known oxygen free radical generator that causes lipid peroxidation and structural alterations in the membrane skeletal protein network, leading to increased membrane rigidity and decreased deformability [5]. Previously, PMS was used in an in vitro study in which either alcohol-free red wine extract (AFRW) or red wine was added to blood followed by incubation with PMS [44]. AFRW provided partial protection from PMS-induced impairment of deformability, while red wine had no preventive influence, thus indicating that AFRW had an anti-oxidative effect. Our results support these previous observations, in that incubation with 500 μM PMS + 0.25% to 2% pure ethanol progressively decreases deformability (i.e., increases SS1/2) when RBC are tested in alcohol-free LORCA media (Figure 3C). Based on these results, it seems reasonable that the presence of ethanol in the red wine portion attenuated the protective effects of polyphenols. In contrast, our ethanol + PMS results showed that the deformability of oxidatively damaged RBC could be improved when ethanol was present in the LORCA media at 0.25% and higher concentrations (Figure 4), presumably acting in a manner similar to the effects of ethanol on normal RBC (Figure 2).
Our aggregation findings (Figure 5A) are consistent with a prior report indicating decreased aggregation when ethanol is added to whole blood [37]; aggregation is also reduced when ethanol is added to a suspension of RBC in 3% 70 kDa dextran (Figure 5B). These results explain why red wine showed a greater inhibitory effect on RBC aggregation compared to alcohol-free red wine extract [44]. Multiple factors can affect RBC aggregation and the reader is referred to more-detailed publications in this area [4] [12] [42]. Decreased aggregation in plasma may be partially due to the ethanol-induced echinocytic shape transformation [36] and to alteration of plasma proteins that promote aggregation (e.g., fibrinogen). Reduced RBC deformability also tends to reduce aggregation [4], yet our results indicate increased cellular deformability (Figure 2). It therefore seems most likely that ethanol-induced changes of the RBC glycocalyx are involved. Based upon the depletion model for aggregation, the extent of a protein or polymer depletion layer near the membrane depends strongly upon the ability of the macromolecule to penetrate the glycocalyx [4] [42]; increased penetration would reduce the depletion layer and hence reduce aggregation. Interestingly, this presumed change of glycocalyx properties is irreversible in that aggregation is also reduced for cells incubated with ethanol but suspended in ethanol-free dextran (Figure 5B).
In summary, ethanol reversibly improves erythrocyte deformability and irreversibly decreases erythrocyte aggregation (Figures 2 and 5), and the presence of ethanol in blood enhances the decrease of RBC deformability due to oxidative stress yet improves the deformability of previously stressed cells (Figures 3 and 4). The specific molecular mechanisms involved require further investigation. Likewise, determining the significance of our results vis-à-vis health benefits due to moderate and frequent wine consumption necessitates additional studies.
Acknowledgments
Miklos Rabai thanks Dr. John C. Wood and Dr. Thomas D. Coates at Children’s Hospital Los Angeles and Dr. Jack Feinberg at University of Southern California for their helpful support in research.
This study was supported in part by SROP-4.2.1.B-10/2/KONV-2010-000 (TAMOP 4.2.1B) by NFL Award RGA006494, NIH Awards HL099412 and HL48484 and by CIRM Award DR1-01452.
References
- 1.Abou-Agag LH, Khoo NK, Binsack R, White CR, Darley-Usmar V, Grenett HE, Booyse FM, Digerness SB, Zhou F, Parks DA. Evidence of cardiovascular protection by moderate alcohol: role of nitric oxide. Free Radic Biol Med. 2005;39:540–548. doi: 10.1016/j.freeradbiomed.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Aikens ML, Grenett HE, Benza RL, Tabengwa EM, Davis GC, Booyse FM. Alcohol-induced upregulation of plasminogen activators and fibrinolytic activity in cultured human endothelial cells. Alc Clin Exp Res. 1998;22:375–381. [PubMed] [Google Scholar]
- 3.Baskurt OK, Hardeman MR, Uyuklu M, Ulker P, Cengiz M, Nemeth N, Shin S, Alexy T, Meiselman HJ. Parameterization of red blood cell shear stress-elongation curves obtained by ektacytometry. Scand J Clin Lab Invest. 2009;69:2299–2309. doi: 10.3109/00365510903266069. [DOI] [PubMed] [Google Scholar]
- 4.Baskurt O, Neu B, Meiselman HJ. Red Blood Cell Aggregation. CRC Press; Boca Raton: 2012. pp. 1–318. [Google Scholar]
- 5.Baskurt OK, Temiz A, Meiselman HJ. Effect of superoxide radicals on red blood cell rheologic properties. Free Rad Med Biol. 1998;24:102–110. doi: 10.1016/s0891-5849(97)00169-x. [DOI] [PubMed] [Google Scholar]
- 6.Bauersachs RM, Wenby RB, Meiselman HJ. Determination of specific red blood cell aggregation indices via an automated system. Clin Hemorheol. 1989;9:1–25. [Google Scholar]
- 7.Beaugé F, Niel E, Hispard E, Perrotin R, Thepot V, Boynard M, Nalpas B. Red blood cell deformability and alcohol dependence in humans. Alcohol Alcohol. 1994;29:59–63. [PubMed] [Google Scholar]
- 8.Booyse FM, Parks DA. Moderate wine and alcohol consumption: beneficial effects on cardiovascular disease. Thromb Haemost. 2001;86:517–528. [PubMed] [Google Scholar]
- 9.Carter C, McGee D, Reed D, Yano K, Stemmermann G. Hematocrit and the risk of coronary heart disease: The Honolulu heart program. Am Heart J. 1983;105:674–679. doi: 10.1016/0002-8703(83)90493-3. [DOI] [PubMed] [Google Scholar]
- 10.Champion HR, Baker SP, Benner C, Fisher R, Caplan YH, Long WB, Cowley RA, Gill W. Alcohol intoxication and serum osmolality. Lancet. 1975;1:1402–1404. doi: 10.1016/s0140-6736(75)92608-2. [DOI] [PubMed] [Google Scholar]
- 11.Chi LM, Wu WG, Sung KL, Chien S. Biophysical correlates of lysophosphatidylcholine- and ethanol-mediated shape transformation and hemolysis of human erythrocytes. Membrane viscoelasticity and NMR measurement. Biochim Biophys Acta. 1990;1027:163–171. doi: 10.1016/0005-2736(90)90080-8. [DOI] [PubMed] [Google Scholar]
- 12.Chien S. Biophysical behavior of red cells in suspensions. In: Surgenor DM, editor. The Red Blood Cell. Academic Press; New York: 1975. pp. 1032–1135. [Google Scholar]
- 13.Chien S. Red cell deformability and its relevance to blood flow. Annu Rev Physiol. 1987;49:177–192. doi: 10.1146/annurev.ph.49.030187.001141. [DOI] [PubMed] [Google Scholar]
- 14.Chin JH, Goldstein DB. Effects of low concentrations of ethanol on the fluidity of spin-labeled erythrocyte and brain membranes. Mol Pharmacol. 1977;13:435–441. [PubMed] [Google Scholar]
- 15.Chmiel BA, Olszowy ZB, Turczynski BB, Kusmierski SA. Effect of controlled ethanol intake on arterial blood pressure, heart rate and red blood cells deformability. Clin Hemorheol Microcirc. 1999;21:325–328. [PubMed] [Google Scholar]
- 16.Cordova AC, Jackson LS, Berke-Schlessel DW, Sumpio BE. The cardiovascular protective effect of red wine. J Am Coll Surg. 2005;200:428–439. doi: 10.1016/j.jamcollsurg.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 17.Dimmitt SB, Rakic V, Puddey IB, Oostryck R, Adams MJ, Chesterman CN, Burke V, Beilin LJ. The effects of alcohol on coagulation and fibrinolytic factors: a controlled trial. Blood Coagul Fibrinolysis. 1998;9:39–45. doi: 10.1097/00001721-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Djoussé L, Gaziano JM. Alcohol consumption and risk of heart failure in the Physicians’ Health Study I. Circulation. 2007;115:34–39. doi: 10.1161/CIRCULATIONAHA.106.661868. [DOI] [PubMed] [Google Scholar]
- 19.Djoussé L, Levy D, Murabito JM, Cupples LA, Ellison RC. Alcohol consumption and risk of intermittent claudication in the Framingham Heart Study. Circulation. 2000;102:3092–3097. doi: 10.1161/01.cir.102.25.3092. [DOI] [PubMed] [Google Scholar]
- 20.El-Sayed MS. Adverse effects of alcohol ingestion post exercise on blood rheological variables during recovery. Clin Hemorheol Microcirc. 2001;24:227–232. [PubMed] [Google Scholar]
- 21.Evans EA. Structure and deformation properties of red blood cells: concepts and quantitative methods. Meth Enzym. 1989;173:3–35. doi: 10.1016/s0076-6879(89)73003-2. [DOI] [PubMed] [Google Scholar]
- 22.Fehr M, Galliard-Grigioni KS, Reinhart WH. Influence of acute alcohol exposure on hemorheological parameters and platelet function in vivo and in vitro. Clin Hemorheol Microcirc. 2008;39:351–358. [PubMed] [Google Scholar]
- 23.Fraser GE, Anderson JT, Foster N, Goldberg R, Jacobs D, Blackburn H. The effect of alcohol on serum high density lipoprotein (HDL): a controlled experiment. Atherosclerosis. 1983;46:275–286. doi: 10.1016/0021-9150(83)90178-8. [DOI] [PubMed] [Google Scholar]
- 24.Grønbaek M, Becker U, Johansen D, Gottschau A, Schnohr P, Hein HO, Jensen G, Sørensen TI. Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann Intern Med. 2000;133:411–419. doi: 10.7326/0003-4819-133-6-200009190-00008. [DOI] [PubMed] [Google Scholar]
- 25.Guillet R, Nalpas B, Perrotin P, Beuzard Y, Koutsouris D, Boynard M. Increased erythrocyte rigidity in chronic alcoholics without cirrhosis: deformability improvement of erythrocyte sub-populations after alcohol withdrawal. Clin Hemorheol. 1991;11:55–62. [Google Scholar]
- 26.Hamazaki T, Shishido H. Increase in blood viscosity due to alcohol drinking. Thromb Res. 1983;30:587–594. doi: 10.1016/0049-3848(83)90267-0. [DOI] [PubMed] [Google Scholar]
- 27.Hannuksela ML, Rämet ME, Nissinen AE, Liisanantti MK, Savolainen MJ. Effects of ethanol on lipids and atherosclerosis. Pathophysiology. 2004;10:93–103. doi: 10.1016/j.pathophys.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Hardeman MR, Goedhart PT, Schut NH. Laser-assisted optical rotational cell analyser (LORCA). II: Red blood cell deformability: elongation index versus cell transit time. Clin Hemorheol. 1994;14:619–630. [Google Scholar]
- 29.Jensen T, Retterstøl LJ, Sandset PM, Godal HC, Skjønsberg OH. A daily glass of red wine induces a prolonged reduction in plasma viscosity: a randomized controlled trial. Blood Coagul Fibrinolysis. 2006;17:471–476. doi: 10.1097/01.mbc.0000240920.72930.63. [DOI] [PubMed] [Google Scholar]
- 30.Johnson RM. Ektacytometry of red blood cells. Meth Enzym. 1989;173:35–80. doi: 10.1016/s0076-6879(89)73004-4. [DOI] [PubMed] [Google Scholar]
- 31.Kannel WB, D’Agostino RB, Belanger AJ. Fibrinogen, cigarette smoking, and risk of cardiovascular disease: insights from the Framingham study. Am Heart J. 1987;113:1006–1010. doi: 10.1016/0002-8703(87)90063-9. [DOI] [PubMed] [Google Scholar]
- 32.Kaul S, Belcik T, Kalvaitis S, Jayaweera AR, Choi SW, Wei K. Effect of modest alcohol consumption over 1–2 weeks on the coronary microcirculation of normal subjects. Eur J Echocardiogr. 2010;11:683–689. doi: 10.1093/ejechocard/jeq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kenyeres P, Rabai M, Toth A, Kesmarky G, Marton Z, Alexy T, Toth K. Reviewing data reduction methods for ektacytometry. Clin Hemorheol Microcirc. 2011;47:143–150. doi: 10.3233/CH-2010-1375. [DOI] [PubMed] [Google Scholar]
- 34.Lowe GDO, Smith WCS, Tunstall-Pedoe HD, Crombie IK, Lennie SE, Anderson J, Barbenel JC. Cardiovascular risk and haemorheology - results from the Scottish heart health study and the MONICA project, Glasgow. Clin Hemorheol. 1988;8:517–524. [Google Scholar]
- 35.McLawhon RW, Marikovsky Y, Thomas NJ, Weinstein RS. Ethanol-induced alterations in human erythrocyte shape and surface properties: modulatory role of prostaglandin E1. J Membr Biol. 1987;99:73–78. doi: 10.1007/BF01870623. [DOI] [PubMed] [Google Scholar]
- 36.Meiselman HJ. Rheologic behavior of shape-transformed human red cells. Biorheology. 1978;15:225–237. doi: 10.3233/bir-1978-153-410. [DOI] [PubMed] [Google Scholar]
- 37.Mesquita R, Gonçalves MI, Dias S, Sargento L, Saldanha C, Martins e Silva J. Ethanol and erythrocyte membrane interaction: a hemorheologic perspective. Clin Hemorheol Microcirc. 1999;21:95–98. [PubMed] [Google Scholar]
- 38.Mohandas N, Chasis JA. Red blood cell deformability, membrane material properties and shape: regulation by transmembrane, skeletal and cytosolic proteins and lipids. Semin Hematol. 1993;30:171–192. [PubMed] [Google Scholar]
- 39.Mohandas N, Evans E. Mechanical properties of the red cell membrane in relation to molecular structure and genetic defects. Ann Rev Biophys Biomed Struct. 1994;23:787–818. doi: 10.1146/annurev.bb.23.060194.004035. [DOI] [PubMed] [Google Scholar]
- 40.Mohandas N, Gallagher PG. Red cell membrane: past, present and future. Blood. 2008;112:3939–3948. doi: 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagai Y, Ishida K, Hirooka M, Nishimaru K. Effect of ethanol on hemorheology in patients with ischemic cerebrovascular disease and elderly healthy men. Clin Hemorheol Microcirc. 2001;25:135–144. [PubMed] [Google Scholar]
- 42.Neu B, Meiselman HJ. Red blood cell aggregation. In: Baskurt OK, Hardeman MR, Rampling MW, Meiselman HJ, editors. Handbook of Hemorheology and Hemodynamics. IOS Press; Amsterdam: 2007. pp. 114–136. [Google Scholar]
- 43.Oonishi T, Sakashita K. Ethanol improves decreased filterability of human red blood cells through modulation of intracellular signaling pathways. Alcohol Clin Exp Res. 2000;24:352–356. [PubMed] [Google Scholar]
- 44.Rabai M, Toth A, Kenyeres P, Mark L, Marton Z, Juricskay I, Toth K, Czopf L. In vitro hemorheological effects of red wine and alcohol-free red wine extract. Clin Hemorheol Microcirc. 2010;44:227–236. doi: 10.3233/CH-2010-1267. [DOI] [PubMed] [Google Scholar]
- 45.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for the coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 46.Renaud SC, Ruf JC. Effects of alcohol on platelet functions. Clin Chim Acta. 1996;246:77–89. doi: 10.1016/0009-8981(96)06228-6. [DOI] [PubMed] [Google Scholar]
- 47.Robinson AG, Loeb JN. Ethanol ingestion-commonest cause of elevated plasma osmolality? N Engl J Med. 1971;284:1253–1255. doi: 10.1056/NEJM197106032842209. [DOI] [PubMed] [Google Scholar]
- 48.Sacco RL, Elkind M, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, Shea S, Paik MC. The protective effect of moderate alcohol consumption on ischemic stroke. JAMA. 1999;281:53–60. doi: 10.1001/jama.281.1.53. [DOI] [PubMed] [Google Scholar]
- 49.Salem RO, Laposata M. Effects of alcohol on hemostasis. Am J Clin Pathol. 2005;123:96–105. doi: 10.1309/113N8EUFXYUECCNA. [DOI] [PubMed] [Google Scholar]
- 50.Taraschi TF, Rubin E. Effects of ethanol on the chemical and structural properties of biologic membranes. Lab Invest. 1985;52:120–131. [PubMed] [Google Scholar]
- 51.Turczyński B, Chmiel B, Słowińska L, Olszowy Z. The influence of controlled ethanol consumption on whole blood and plasma viscosity. Wiad Lek. 2001;54:409–417. [PubMed] [Google Scholar]
- 52.Vaya A, Falco C, Fernandez P, Contreras T, Valls M, Aznar J. Erythrocyte aggregation determined with the Myrenne aggregometer at two modes (M0, M1) and at two times (5 and 10 sec) Clin Hemorheol Microcirc. 2003;29:119–127. [PubMed] [Google Scholar]
- 53.Widmer J, Raffin Y, Gaillard JM, Tissot T. In vitro effects of short-chain aliphatic alcohols, benzyl alcohol and chlorpromazine on the transport of precursors of monoamines across the human erythrocyte membrane. Neuropsychobiology. 1987;18:60–67. doi: 10.1159/000118394. [DOI] [PubMed] [Google Scholar]