FIG. 1.
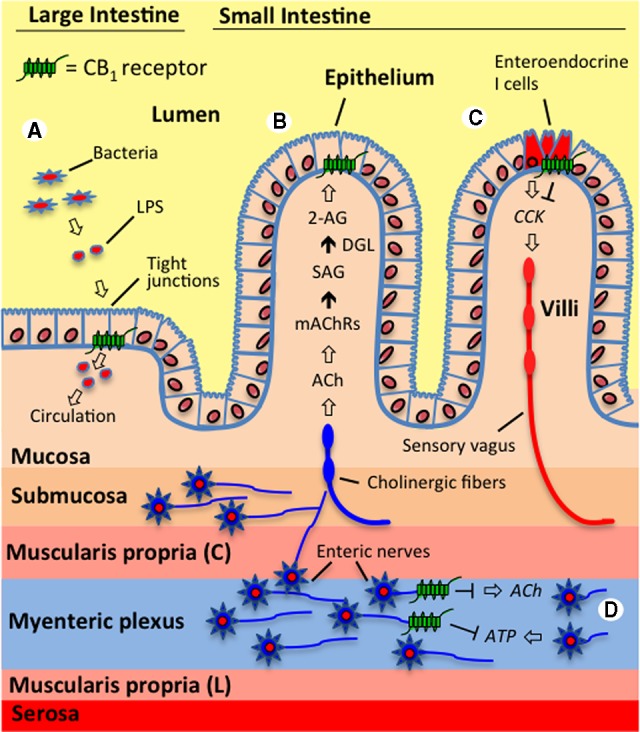
The endocannabinoid system controls a variety of gastrointestinal functions. (A) The endocannabinoid system in the large intestine is proposed to interact with gut microbiota and regulate epithelial barrier permeability. For example, activating cannabinoid type 1 receptors (CB1Rs) in mice increased circulating levels of lipopolysaccharide (LPS)—which is an endotoxin released from Gram-negative bacteria—through a proposed mechanism that includes decreased expression of the tight junction proteins, occludin and zonula occludens-1, and resulting increases in permeability.76 It is suggested that CB1Rs located in the intestinal epithelium control these processes. (B) Endocannabinoid signaling in the jejunum mucosa of the small intestine is triggered by fasting and tasting dietary fats and is proposed to be a general hunger signal that acts at local CB1Rs to inhibit satiation.42,43 The evidence suggests that during fasting, cholinergic signaling (acetylcholine, ACh)—possibly by the efferent vagus nerve—activates muscarinic acetylcholine receptors (mAChRs) in the small intestine, which, in turn, drive the conversion of the 2-arachidonoyl-sn-glycerol (2-AG) precursor, 1-stearoyl-2-arachidonoyl-sn-glycerol (SAG), into 2-AG through the activity of diacylglycerol lipase (DGL). Inhibiting subtype m3 mAChRs locally in the rat intestine blocked fasting-induced production of 2-AG in the jejunum mucosa and inhibited refeeding after a 24-h fast to the same levels as when a peripherally restricted CB1R antagonist was administered.43 (C) Endocannabinoid activity at CB1Rs located on small intestinal enteroendocrine I cells—which produce and secrete the peptide, cholecystokinin (CCK)—is suggested to promote feeding during a fast and drive the intake of fat-rich foods by inhibiting the release of CCK, which normally binds CCK receptors on the sensory vagus nerve and induces satiation after a meal.42,43 Supporting this hypothesis, the expression of CB1R mRNA on CCK-containing enteroendocrine I cells in the mouse small intestine has been reported,59 which suggests that CB1Rs in the gut mucosa control feeding by inhibiting the release of CCK and therefore indirectly modifying the activity of the sensory vagus. (D) Many studies provide evidence that CB1Rs on enteric nerves control intestinal contractility by inhibiting the release of the excitatory neurotransmitter, ACh.1 Recent studies also suggest that contractility is controlled by a dynamic interplay between the retrograde messengers, the endocannabinoids, and purines (e.g., adenosine triphosphate, ATP), which act in an opposing manner. It is proposed that the excitatory actions on contractility for ATP are mediated through increases in ACh, which are inhibited by the activation of prejunctional CB1Rs on enteric nerves.33,34 Both systems may functionally interact to regulate synaptic strength in the enteric nervous system.
