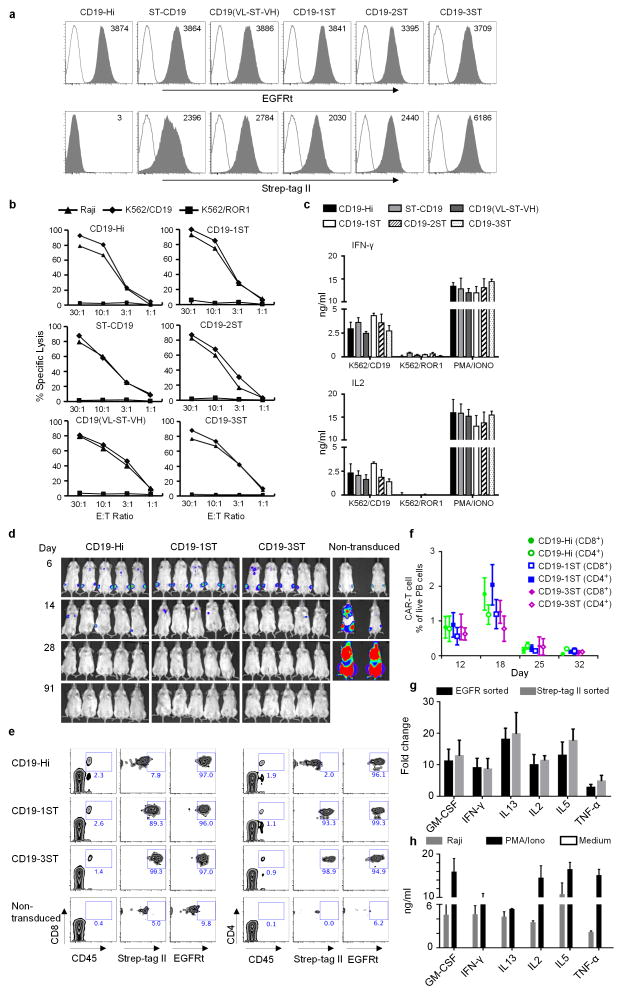
Figure 1. Expression and function of CD19 CARs that contain Strep-tag II.
(a) Analysis of CD19 CAR expression. Primary human CD8+ T cells were transduced with epHIV7 lentiviral vectors encoding a CD19-Hi CAR or CD19 CARs with Strep-tag in various extracellular locations. Each CAR contained a 4-1BB/CD3ζ intracellular signaling domain and EGFRt downstream of a T2A element. Transduced cells were sorted for EGFRt+ cells by fluorescence-activated cell sorting (FACS), and purity confirmed by staining with anti-EGFR (grey – top panels). Cell surface expression of the Strep-tag CARs was evaluated by staining with anti-Strep-tag II antibodies (grey - bottom panels). Non-transduced cells (white) served as controls for staining. (b) Cytolytic activity of CD19-Hi and Strep-tag 4-1BBζ CAR-T cells. After sorting for EGFRt expression, CD8+ T cells transduced with each of the CARs were tested for lysis of CD19+ Raji lymphoma and K562 leukemia transduced with CD19 (K562/CD19) or ROR1 (K562/ROR1) at various effector/target (E:T) ratios.
(c) IFN-γ and IL2 production by CD19-Hi and Strep-tag 4-1BBζ CAR-T cells 24h after stimulation with K562/CD19 and K562/ROR1. PMA/Ionomycin treated T cells were used as a positive control. The data in a–c is representative of three experiments with CD8+ T cells from different donors.
(d) Cohorts of NSG mice were inoculated with 0.5×106 firefly luciferase expressing CD19+ Raji lymphoma cells (Raji-ffluc) via tail vein injection and treated 7 days after tumor inoculation with 2.5×106 CD19 4-1BBζ CAR-T cells with Hi, 1ST, and 3ST spacers, respectively. CAR-T cells were formulated in a CD8:CD4 ratio of 1:1. Tumor progression and distribution were evaluated by serial bioluminescence imaging.
(e) Tracking CAR-expressing T cells in vivo by staining with anti-Strep-tag II mAb. Blood obtained from mice 8 days after the T cell infusions was stained with anti-human CD45, CD8, CD4, anti-Strep-tag II and anti-EGFR mAbs, and analyzed by flow cytometry. Expression of Strep-tag II and EGFRt on CD45+ CD8+ and CD45+ CD4+ T cells is shown.
(f) Kinetics of expansion and contraction of CD19 CAR-T cells in the blood after adoptive transfer to NSG mice bearing Raji tumors. The mean frequency of CD45+CD8+ EGFRt+ and CD45+CD4+ EGFRt+ human T cells in blood of the mice (n=5) of each group at various times after the T cell infusion is shown. The data in d–f are representative of three experiments.
(g) Fold-change in expression of selected cytokine genes in CD19-1ST/4-1BBζ CAR-T cells after infusion to Raji tumor bearing and non-tumor bearing NSG mice. CAR-T cells were sorted 2 days after infusion from blood, bone marrow and spleen after staining with anti-EGFR or anti-Strep-tag mAb. Gene expression was analyzed using a human common cytokine PCR array. The mean fold change values of cytokine genes in the sorted CAR-T cells from NSG/Raji vs sorted CAR-T cells from non-tumor bearing NSG mice were calculated. Samples were run in triplicate and data are presented as the mean fold increase ± SD.
(h) Cytokine production by CD8+ T cells expressing CD19-1ST/4-1BBζ CARs 2 days after stimulation with Raji cells in vitro. Supernatants of CAR-T cells co-cultured with CD19+ Raji cells (intra-assay triplicates) for 48 h were pooled together and analyzed using the Luminex Multiplex platform to validate the production of cytokines that were upregulated in CAR-T cells in NSG mice bearing Raji tumors. PMA/Ionomycin treated and non-treated T cells were used as positive and negative control, respectively. The results are representative of biological replicates.