Fig. 3.
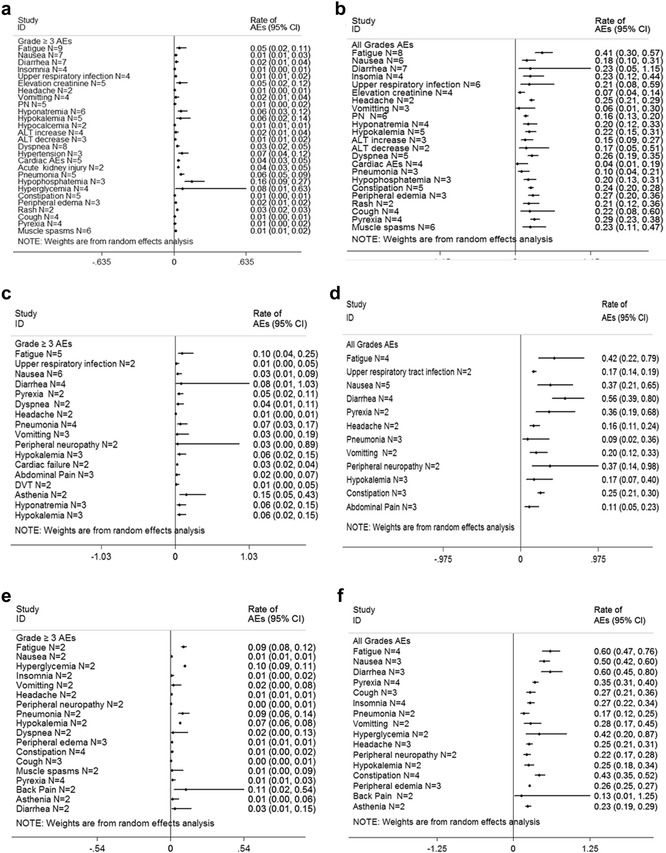
Meta-analysis of nonhematologic adverse events (AEs) with variable carfilzomib/panobinostat/elotuzumab-containing combination regimens in patients with multiple myeloma. a ≥Grade 3 nonhematologic AEs with carfilzomib combination regimens in patients with relapsed and refractory multiple myeloma. b All grades nonhematologic AEs with carfilzomib combination regimens in patients with relapsed and refractory multiple myeloma. c ≥Grade 3 nonhematologic AEs with panobinostat combination regimens in patients with relapsed and refractory multiple myeloma. d All grades nonhematologic AEs panobinostat combination regimens in patients with relapsed and refractory multiple myeloma. e ≥Grade 3 nonhematologic AEs with elotuzumab combination regimens in patients with relapsed and refractory multiple myeloma. f All grades nonhematologic AEs with elotuzumab combination regimens in patients with relapsed and refractory multiple myeloma. N number of the included trials, CI 95 % confidence interval, Random random effects model
