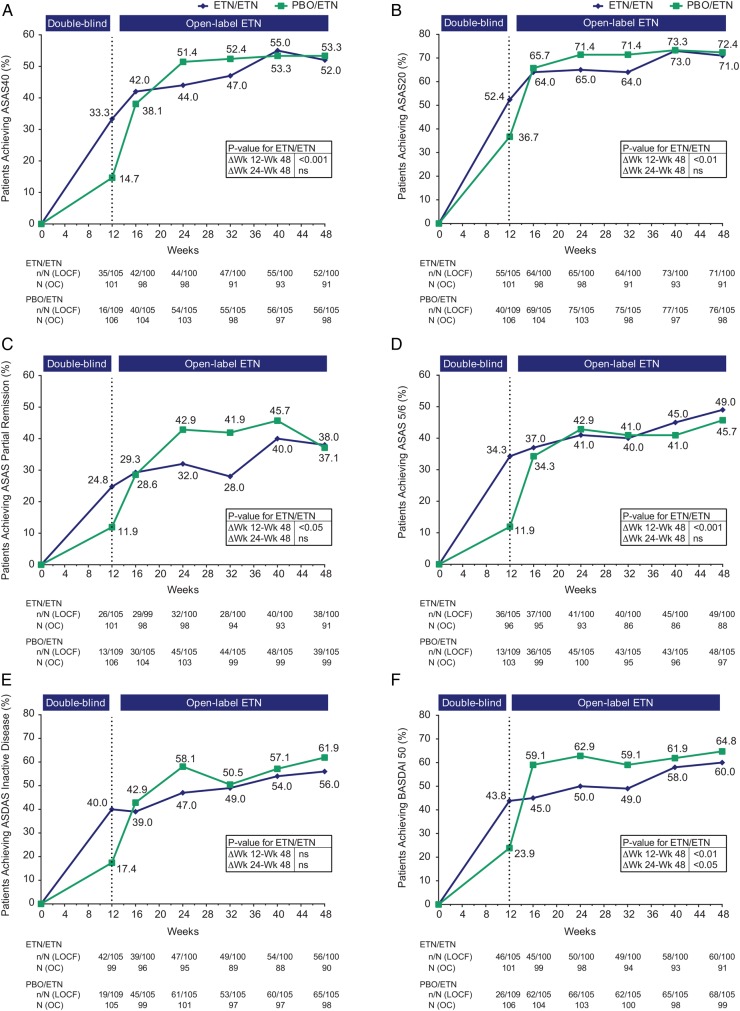
Figure 2.
Proportion of patients achieving (A) ASAS40 response, (B) ASAS20 response, (C) ASAS partial remission, (D) ASAS 5/6, (E) ASDAS inactive disease and (F) BASDAI50. Population is modified intention-to-treat (mITT), last observation carried forward (LOCF). The actual number of patients, observed case (OC), is also shown. p Values for differences in results between weeks 12 and 48 and between weeks 24 and week 48 for the ETN/ETN group are from McNemar’s test, OC data. ASAS, Assessment of SpondyloArthritis international Society; ASDAS, Ankylosing Spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; ETN, etanercept; ns, non-significant; PBO, placebo; Δ, change.