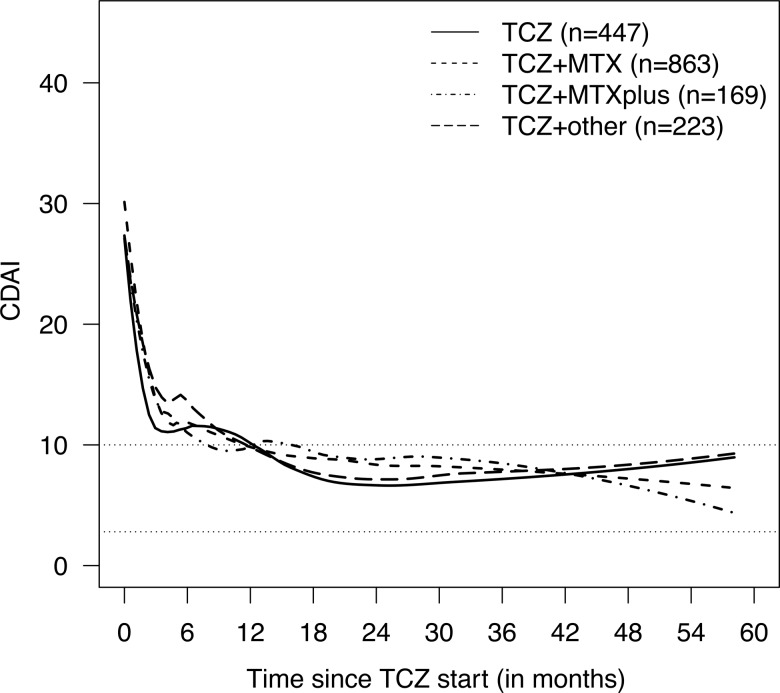
Figure 1.
Smoothed time courses of Clinical Disease Activity Index (CDAI) by tocilizumab (TCZ) treatment. The data represent all 1702 eligible patients with at least one CDAI value totalling 9943 observations. Data were smoothed separately for each TCZ treatment using local quadratic regression. Treatment groups ‘TCZ’, ‘TCZ+ methotrexate (MTX)’, ‘TCZ+MTXplus’, and ‘TCZ+other’ represent TCZ as monotherapy and in combination with MTX, MTX+other synthetic disease-modifying antirheumatic drugs (sDMARD(s)), and at least one sDMARD other than MTX, respectively. Numbers of patients providing CDAI information beyond 12, 24, 36 and 48 months were 162, 76, 32 and 7 for ‘TCZ’, 427, 262, 133 and 41 for ‘TCZ+MTX’, 80, 41, 21 and 11 for ‘TCZ+MTXplus’, and 90, 55, 27 and 11 for ‘TCZ+other’, respectively. Of note, all Swedish patients were excluded due to lack of a global physician's assessment of disease in this registry.