Figure 1.
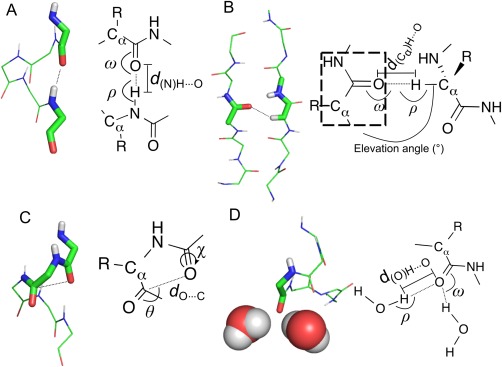
Backbone‐carbonyl‐oxygen non‐covalent interaction (NCIC=O) considered in this analysis. (A) “Standard” hydrogen bonds, as exemplified by NHi→C=Oi−4 hydrogen bonds found in an α‐helix (NHbb, dNH⋯O ≤ 2.44 Å25; ω ≥ 90°; ρ ≥ 90°). Other donor groups include (i) side‐chain NH, e.g., from lysine or arginine, (NHsc, parameters as for NHbb); and (ii), side‐chain hydroxyl groups (OHsc, d(O)H⋯O ≤ 2.31 Å25; ω ≥ 90°; ρ ≥ 90°). (B) Hydrogen bonds with a Cα—H group donor (CαH, d(Cα)H⋯O ≤ 2.68 Å25; ω ≥ 90°; ρ ≥ 90°, elevation angle < 50°), or alternatively donated by other methyl or ethyl groups from protein sidechains9 (CHX, parameters as for CαH). (C) n→π* interactions, shown with a main‐chain carbonyl group acceptor (dC⋯O ≤ 3.22 Å; 95° ≥ θ ≥ 125°; Cα⋯C⋯O⋯H dihedral χ ≥ 120°57); but these can also have a side‐chain acceptor, e.g., asparagine or glutamine, (n→π* sc, parameters as for n→π*). (D) Hydrogen bonds made with water (HOH, d(O)H⋯O ≤ 2.31 Å25; ω ≥ 90°; ρ ≥ 90°).
