Figure 2.
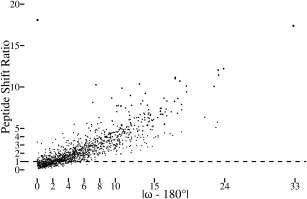
Significant atomic shifts are caused by tight ω restraints. Plotted for each peptide in the dozen test structures is the peptide shift ratio (defined below) as a function of the degree to which ω deviates from 180° in the standard refinement. For each atom in a structure refined using standard restraints, the standard uncertainty in the position of each atom4 was estimated using the Online_DPI webserver.26 Also, the shift for each atom between the standard vs. tight ω refined structures was calculated. Noting that the tight restraints often lead mostly to shifts in the central C, O, and N atoms of a peptide (e.g. Fig. 1), we defined a “peptide shift ratio” for each peptide as the rms of the shifts of the three central atoms in the peptide (i.e. Oi‐1, Ci‐1, and Ni) divided by the rms of the estimated standard uncertainties of the same three atoms. A value of 1 means that the rms shift in the atom positions is equal to the uncertainty in the positions of those atoms. The most nonplanar residue in the dataset is the 2CWS 189‐190 peptide shown in Figure 1. The one outlier in the plot is the 179 to 180 peptide from PDB entry 3QL9 with an ω angle in the standard refinement that is only 0.1° from planar but for which the backbone oxygen shifts ∼0.4 Å to yield a peptide shift ratio of ∼18. This can be rationalized in that this peptide oxygen has high anisotropy and that the method used to estimate the positional uncertainty does not take the anisotropy into account.26
