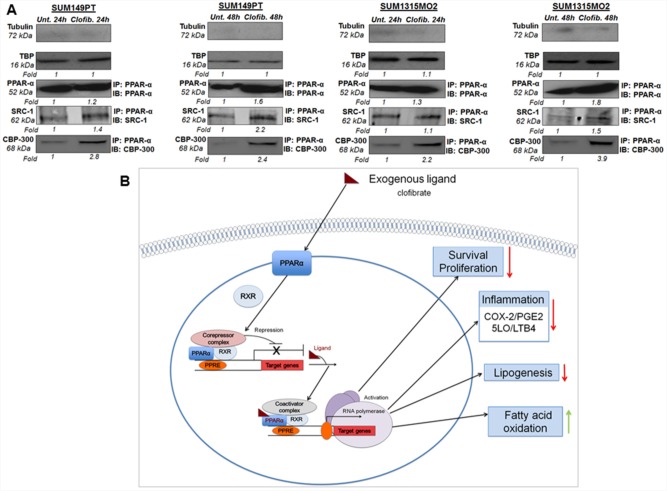
Figure 12. A proposed model for the role of PPARα agonist clofibrate in the regulation of inflammatory and lipid pathways in breast cancer cells.
A. Effect of clofibrate treatment on coactivator proteins in the nuclear complexes of SUM149PT and SUM1315MO2 cells. Nuclear complexes were prepared from SUM149PT and SUM1315MO2 that were untreated or treated with 20 μM clofibrate for 24 h and 48 h and immunoprecipitated with PPARα antibody, and Western blotted for SRC-1 and p300/CBP, and normalized with respect to the levels of total protein levels. Tubulin was used as the loading control. B. The present study indicates that highly metastatic form of breast cancer cell lines including SUM149PT and SUM1315MO2 cells express tremendous level of PPARα along with abundant expression and activity of inflammatory pathways of COX-2 and 5-LO. We demonstrated that SUM149PT and SUM1315MO2 cells are metabolically active, express the active form of FASN, and secrete free fatty acids in their tumor microenvironment and FASN/lipogenic pathways rich phenotype. Our study unraveled that PPARα activation in breast cancer cells downregulated COX-2 and 5-LO inflammatory pathways. Clofibrate treatment reduced lipogenic enzymes (FASN) and induced enzymes involved in fatty acid oxidation (CPT-1a and SREBP-2). PPARα activation via clofibrate showed anti-proliferative effects in breast cancer cells via inhibition of survival kinases (NF-kB and ERK1/2), cell cyclin kinases, and induction of p21. Clofibrate treatment modulated the expression of PPRE containing target genes via induction of coactivators (SRC-1 and p300/CBP) in nuclear complexes probably binding to PPARα. Red arrow represents inhibition and green arrow represents induction in the schematic.