Figure 5. The KH34 domain of IMP1 is required for the target mRNA binding and for repressing proliferation of carcinoma cells as well as the growth of cell-derived breast tumors.
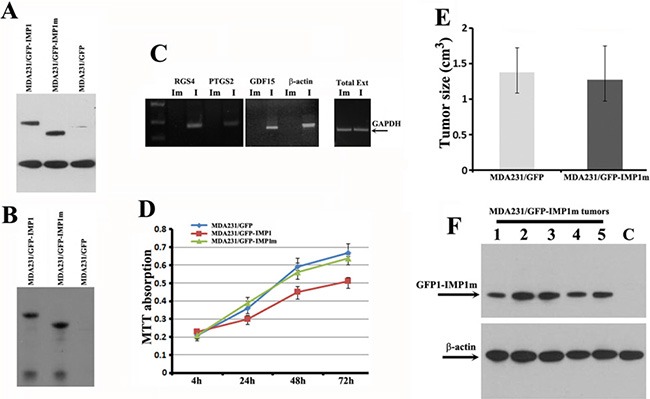
(A) Western blots showing the expression of IMP1 and IMP1 truncate in MDA231/GFP-IMP1 and MDA231/GFP-IMP1m lines. (B) Co-IP experiments were performed in the extracts prepared from MDA231 cell lines using antibodies against Flag-tag. Western blots indicated that the Flag-tagged GFP-IMP1 and GFP-IMP1m were successfully precipitated. (C) Co-precipitated RNAs were extracted and used for RT-PCR assays. GDF15, RGS4, PTGS2 and β-actin mRNAs were not precipitated when the KH34 domain of IMP1 was deleted. GAPDH mRNA was used as an internal control for the extracts used in the experiments. Im: MDA231/GFP-IMP1m cells. I: MDA231/GFP-IMP1 cells. (D) MTT assays were used to measure the rate of cell proliferation. Loss of RNA binding activity by deletion of KH34 domain abolished the ability of IMP1 in repressing proliferation of MDA231 cells (P < 0.005). Bars indicate standard error of mean from three independent experiments, (P < 0.01). (E) Average tumor volumes in MDA231/GFP and MDA231/GFP-IMP1m cell-derived xenograft animals, P > 0.5. (F) Western blots indicated the expression of GFP-IMP1m in MDA231/GFP-IMP1m cell-derived xenografts (Lanes 1–6). Lane C was a negative control for the xenograft derived from MDA231/GFP cells. Antibody for β-actin was used as a loading control.
