Figure 2. Effects of hnRNP A1 and A2 depletion on cell proliferation, anchorage-independent growth, apoptosis, and EGFR signaling.
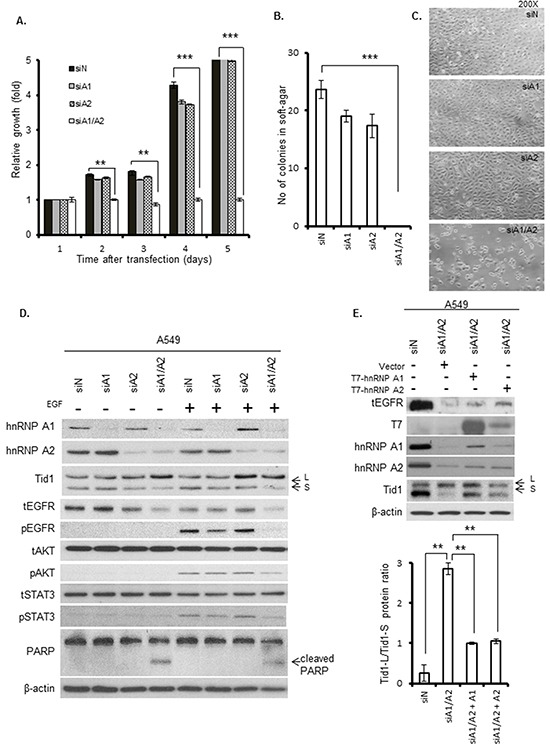
A549 cells were transfected with siA1, siA2, siA1/A2, or control siN for 72 h, and then cultured for the indicated durations in the absence of transfection reagents. A. Cell proliferation was determined by MTT assay. B. Anchorage-independent growth was determined by plating the transfected cells in soft agar, culturing the cells for 2 weeks, and scoring the number of colonies. Symbol: ***, p < 0.001 based on the Student's t-test. C. Microscopic morphology of cells after transfection for 72 h. D. Immunoblotting of cell lysates with the indicated primary antibodies. The transfected cells were cultured in the absence of serum for 24 h, and then incubated in the presence or absence of 50 ng/mL EGF at 37°C for 15 min. The total and phosphorylated forms of EGFR, STAT3 and AKT are indicated by the prefixes “t” and “p”, respectively. The Tid1-L and Tid1-S splicing variants are indicated with arrows labeled “L” and “S,” respectively. β-Actin was used as an internal control. E. A549 cells were transfected with siA1/A2, or control siN. After 24 h, the transfection reagents were removed and the cells were cultivated in regular medium for 4 h. The hnRNP A1/A2 depleted cells were then transfected with plasmids expressing T7-tagged hnRNP A1 (T7-hnRNP A1), hnRNP A2 (T7-hnRNP A2) or the empty vector (Vector). After 24 h, the transfection reagents were removed and the transfected cells were cultured for additional 24 h in regular medium before being analyzed for the expression of proteins by Western blot (top panel). The levels of Tid1-L and Tid1-S were quantified by densitometry, and the Tid1-L/Tid1-S ratios from three independent experiments are shown in bottom panel.
