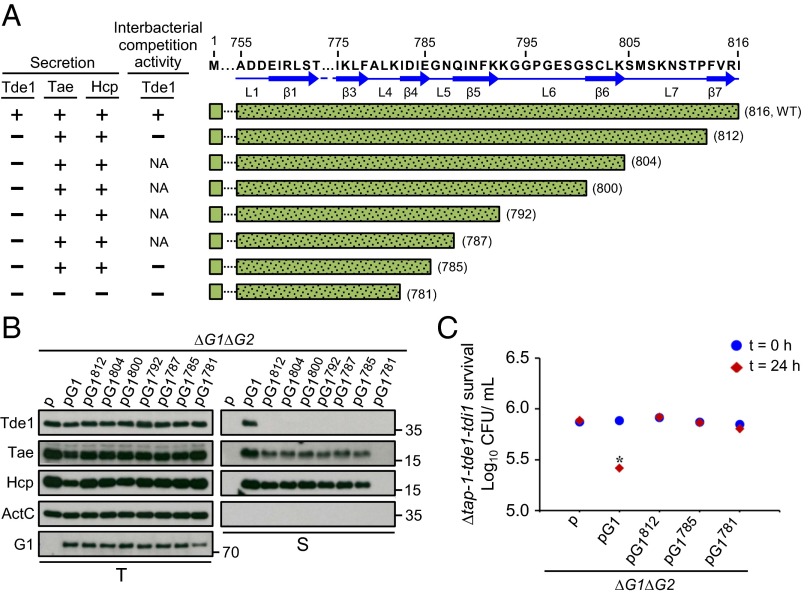
Fig. 3.
Effect of VgrG1 C-terminal truncation on type VI secretion and Tde1-dependent interbacterial competition activity. (A) Amino acid sequence of VgrG1 C-terminal extension shown with the indicated residue number and predicted β-strands and loops marked with blue solid arrows and lines, respectively. VgrG1 C-terminal truncated variants are shown with green bars filled with dots, and the number in parentheses represents the terminal amino acid of each variant. Presence (+) or absence (−) of Tde1, Hcp, and Tae secretion and Tde1-dependent antibacterial activity is based on Fig. 4 B and C. NA: not analyzed. (B) Western blot analysis of total (T) and secreted (S) proteins from A. tumefaciens ∆vgrG1∆vgrG2 expressing the plasmid control (p) or indicated plasmid. Protein names and molecular weight markers are at the left and right, respectively. The soluble ActC protein was used as an internal nonsecreted protein control. (C) Effect of VgrG1 C-terminal truncation on Tde1-dependent interbacterial competition activity. Various A. tumefaciens strains shown on the x axis were mixed with ∆tap-1-tde1-tdi1 at a 10:1 ratio and infiltrated into N. benthamiana leaves. The survival of ∆tap-1-tde1-tdi1 collected at 0 and 24 h was quantified as cfu. Data are mean ± SEM (n = 5 biological repeats from two independent experiments). Significant difference compared with ∆vgrG1∆vgrG2 (∆G1∆G2) at 24-h postinfiltration (*P ≤ 0.01). vgrG1 and vgrG2 are abbreviated as G1 and G2, respectively.