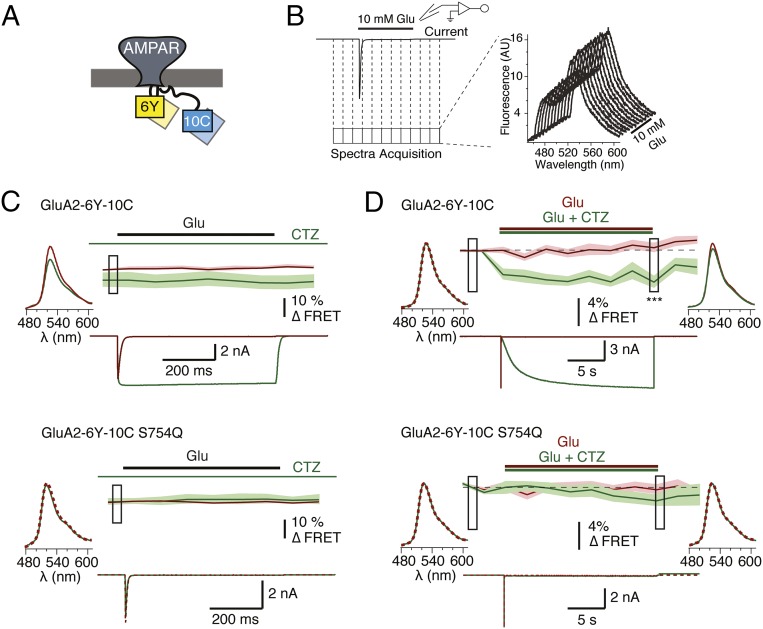
Fig. 3.
Characterization of the GluA2-6Y-10C receptor FRET by voltage-clamp fluorometry. (A) Cartoon representation of a dual-tagged AMPAR with YFP inserted in the intracellular loop between M1 and M2 transmembrane helices and CFP inserted in the C-terminal tail. (B) Schematic representation of the protocol used for simultaneous acquisition of fluorescence spectra and current, and a representative train of collected spectra. (C) Averaged FRET traces, with the SD indicated by pale shading, and representative time-locked current traces from cells expressing GluA2-6Y-10C (6Y-10C; Upper, n = 8) and the corresponding S754Q mutant (6Y-10C-S754Q; Lower, n = 5). Traces were recorded in the presence and absence of CTZ (green and red traces, respectively). Ratiometric FRET was calculated as described in Materials and Methods from emission spectra obtained at 10 Hz. The data were normalized on a frame-by-frame basis to the control condition (absence of CTZ) to allow comparison of the equilibrium values. Averaged acceptor emission spectra following donor excitation at 445 nm are shown for both conditions from the point during the recording indicated by the box. (D) Average spectral FRET recording of GluA2-6Y-10C (Upper, n = 20) and GluA2-6Y-10C S754Q (Lower, n = 19) constructs in a total of 18 frames during a prolonged (20 s) exposure to glutamate (red traces) or glutamate and CTZ (green traces). Due to the limited speed of fluorescence recording for FRET experiments (0.5 Hz), the time course of CTZ-induced FRET decrease was not resolved. The data were normalized to the baseline before the jump. Boxes indicate samples compared between green and red traces with an unpaired two-tailed Student’s t test (P = 8E-05 for the last sample during CTZ application). The averaged acceptor emission spectra for the boxed regions collected in the presence (green) and absence (red) of CTZ are shown for both the GluA2-6Y-10C and the S754Q mutant.