Abstract
Please cite this paper as: Shoji et al. (2011) A plant‐based system for rapid production of influenza vaccine antigens. Influenza and Other Respiratory Viruses 6(3), 204–210.
Background Influenza virus is a globally important respiratory pathogen that causes a high degree of annual morbidity and mortality. Significant antigenic drift results in emergence of new, potentially pandemic, virus variants. The best prophylactic option for controlling emerging virus strains is to manufacture and administer pandemic vaccines in sufficient quantities and to do so in a timely manner without impacting the regular seasonal influenza vaccine capacity. Current, egg‐based, influenza vaccine production is well established and provides an effective product, but has limited capacity and speed.
Objectives To satisfy the additional global demand for emerging influenza vaccines, high‐performance cost‐effective technologies need to be developed. Plants have a potential as an economic and efficient large‐scale production platform for vaccine antigens.
Methods In this study, a plant virus‐based transient expression system was used to produce hemagglutinin (HA) proteins from the three vaccine strains used during the 2008–2009 influenza season, A/Brisbane/59/07 (H1N1), A/Brisbane/10/07 (H3N2), and B/Florida/4/06, as well as from the recently emerged novel H1N1 influenza A virus, A/California/04/09.
Results The recombinant plant‐based HA proteins were engineered and produced in Nicotiana benthamiana plants within 2 months of obtaining the genetic sequences specific to each virus strain. These antigens expressed at the rate of 400–1300 mg/kg of fresh leaf tissue, with >70% solubility. Immunization of mice with these HA antigens induced serum anti‐HA IgG and hemagglutination inhibition antibody responses at the levels considered protective against these virus infections.
Conclusions These results demonstrate the feasibility of our transient plant expression system for the rapid production of influenza vaccine antigens.
Keywords: Antibody, influenza, plant‐based, recombinant hemagglutinin, subunit vaccine
Introduction
Seasonal influenza continues to be an unavoidable public health threat causing an estimated 250 000–500 000 deaths worldwide and 30 000–50 000 deaths in the United States annually. 1 Influenza outbreaks caused by different strains of types A and B viruses are usually the result of antigenic changes in the surface glycoproteins of the virus, enabling the new variants to escape immunity generated by vaccination or infection by previously circulating strains. In fact, significant antigenic drift is often associated with influenza epidemics, including those caused by A/Sydney/05/97‐like strains in 1997 and the A/Fujian/411/02‐like strains in 2003. 2 , 3 While vaccination remains the most effective tool to fight against influenza epidemics, this continual antigenic drift requires vaccines to be reformulated on an annual basis. 2 Currently, most manufacturers use embryonated eggs to produce influenza vaccines. However, there are some disadvantages in using this vaccine substrate. For example, occasionally, a particular virus strain recommended for vaccine production, such as A/Fujian/411/02 or some of influenza B viruses, 2 , 4 does not replicate well in eggs resulting in vaccine shortage.
On June 11, 2009, the World Health Organization raised the global alert level to Phase 6, the Pandemic phase, in response to the emergence and global spread of the novel H1N1 influenza A virus that appears to result from reassortment among human, avian, and swine viruses. Although the novel H1N1 virus belongs to the subtype prevalent for the most of the 20th century, only a fraction of the population (elderly) had some preexisting immunity, 5 suggesting an unpredictable nature of changes that may lead to altered pathogenicity of virus. To date, the novel H1N1 virus has spread to 214 countries worldwide where laboratory‐confirmed human cases of infection with over 18 449 deaths have been reported. 6 The speed by which this virus spread and the potential devastation it may have caused make the capability for rapid development and manufacturing of a pandemic influenza vaccine in sufficient quantities a global priority. The development of novel substrates and approaches such as cell culture or recombinant protein‐based vaccines is a promising step toward the rapid, large‐scale production of both pandemic and seasonal vaccines.
During the past decade, plants have emerged as an economic and efficient large‐scale production platform for vaccine antigens. 7 , 8 More specifically, plant virus‐based transient expression systems show further promise for producing high levels of target in a short period of time. 9 , 10 We have previously described a novel transient expression system for the production of vaccine antigens in plants – a ‘launch vector’ system that combines the elements of plant RNA viral vectors and an Agrobacterium binary plasmid. 11 This approach enables uniform, high levels of target protein expression and rapid scale‐up of production. Vaccine antigens produced in this system have been shown to elicit protective immune responses in animal models. 12 , 13
Here, we demonstrate the potential of our transient plant expression system to produce hemagglutinin (HA) proteins from the influenza strains comprising the 2008–2009 seasonal vaccine (A/Brisbane/59/07, A/Brisbane/10/07, and B/Florida/4/06) and from the novel H1N1 influenza A strain (A/California/04/09), and evaluate immunogenicity of these plant‐produced proteins in mice.
Materials and methods
Ethics statement
All animal protocols were approved by the University of Delaware Institutional Animal Care and Use Committee under Animal Use Protocol Number 1173.
Cloning and expression of HA antigens in plants
The HA sequences, encompassing amino acids 18–529 of the A/Brisbane/59/07, 17–529 of the A/Brisbane/10/07, 15–547 of the B/Florida/4/06 or 17–530 of the A/California/04/09 strains of influenza viruses (accession number ACA28844, ABW23353, ACA33493 or ACP41105, respectively), were optimized for expression in plants and synthesized by GENEART AG (Regensburg, Germany) as described previously. 12 Each optimized HA sequence was then inserted into the launch vector pGRD4 as described elsewhere. 12 The pGRD4 vector carrying the target sequence was introduced into the Agrobacterium tumefaciens strain GV3101 along with pSoup that provides replication functions in trans. 14 The resulting bacteria were then grown in a minimal medium overnight at 28°C. The optical density of the Agrobacterium culture was determined and adjusted to approximately 0·5. The Agrobacterium culture was introduced by hand infiltration into the aerial parts of 6‐week‐old soil‐grown Nicotiana benthamiana plants as described previously. 15 The expression levels of a target protein in plant leaves and its solubility were monitored daily from 5 to 7 days post‐infiltration (DPI) by Western blot analysis using an anti‐hexa‐histidine (6xHis) tag mouse monoclonal antibody (mAb) (Roche Applied Science, Indianapolis, IN, USA). The image was taken using the GeneSnap software on a GeneGnome and quantified using the Gene Tools software (Syngene Bioimaging, Frederick, MD, USA). The day of the maximum expression was determined for production purposes. Scale‐up infiltration of hydroponic trays of N. benthamiana was then performed by vacuum as described previously, 15 and the tissue was harvested at the time of peak expression for purification. A schematic diagram of the target protein production in N. benthamiana using the launch vector system is shown in Figure 1.
Figure 1.
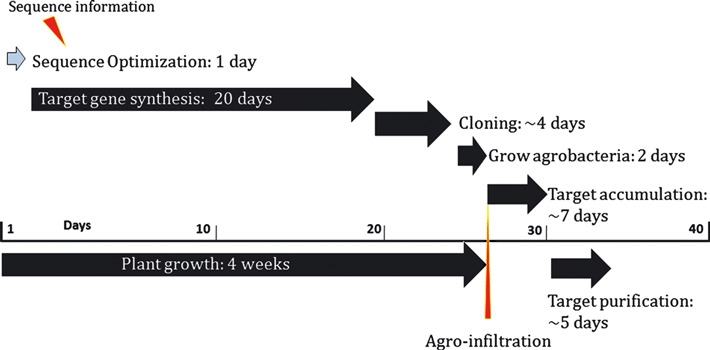
Schematic diagram of the target protein production in N. benthamiana using the launch vector pGRD4. The diagram shows production flow and time course after obtaining the amino acid sequences of target antigens.
Purification and in vitro characterization of plant‐produced HA antigens
Aerial tissues of N. benthamiana expressing each HA antigen were harvested at 7 DPI and then frozen at −80°C until the day of purification. The frozen tissues were mechanically homogenized and incubated with 0·5% Triton X‐100. The crude extracts were then clarified by centrifugation (78 000 g for 30 min) and microfiltration. After clarification, the extracts were initially purified using immobilized metal affinity chromatography (Ni‐sepharose; GE Healthcare, Piscataway, NJ, USA) with Ni buffer A (50 mm sodium phosphate, 500 mm NaCl, 20 mm imidazole, pH 7·5) as a binding and washing buffer and 60% Ni buffer B (Ni buffer A with 500 mm imidazole) as an elution buffer. Further purification was carried out by anion‐exchange chromatography (Capto Q; GE Healthcare) with Q buffer A (10–50 mm sodium phosphate, pH 5·8) as a loading buffer on a Bio‐Rad Duo Flow system (Bio‐Rad Laboratories, Hercules, CA, USA) using the Biologics software. Purified antigens were then dialyzed against phosphate‐buffered saline (PBS) and quantified by absorbance of UV at 280 nm. Purified HA antigens were separated on 10% SDS–PAGE followed by staining with Coomassie blue (Pierce, Rochford, IL, USA) for analysis of their molecular weight and purity. The purified HA antigens from A/Brisbane/59/07, A/Brisbane/10/07, B/Florida/4/06, and A/California/04/09 were designated as HAB1(H1), HAB1(H3), HAF1(B), and HAC1, respectively.
Immunogenicity of plant‐produced HA antigens in mice
Groups of 6‐week‐old Balb/c mice, 6 mice per group, were immunized with HAB1(H1), HAB1(H3), or HAF1(B) subcutaneously (s.c.) at 2‐week intervals at days 0, 14, and 28. Three different antigen doses were tested: 60, 30, and 15 μg in 50 μl of PBS. All immunizations included 10 μg of Quil A as an adjuvant (Accurate Chemicals, Westbury, NY, USA). Serum samples were collected prior to each immunization and 2 weeks after the third dose. Animals in control groups received PBS with 10 μg of Quil A. In another study, groups of Balb/c mice were immunized with HAC1 twice at a 3‐week interval (days 0 and 21) either s.c. with doses of 30, 15, or 5 μg with 10 μg of Quil A or intramuscularly (i.m.) with a dose of 30 μg absorbed to Alhydrogel (1:20 w/w) (Accurate Chemicals, Westbury, NY, USA). As in the first study, animals in control groups received PBS with 10 μg of Quil A. Serum samples were collected prior to each immunization, as well as 10 and 21 days after the second immunization. Serum IgG and hemagglutination inhibition (HI) antibody titers were determined as described later.
Assessment of serum antibody titers
Serum IgG titers were measured using enzyme‐linked immunosorbent assay (ELISA) using 96‐well MaxiSorp plates (NUNC, Rochester, NY, USA) coated with inactivated A/Brisbane 59/07 (NIBSC 07/346), A/Brisbane/10/07 (TGA 2007/79B, Australia), or B/Florida/4/06 (NIBSC 08/140) virus as described previously. 12 For the IgG‐specific ELISA of serum samples from HAC1‐immunized mice, A/California/07/09 × 179A (CDC # 2009713114, Georgia AT) was grown in embryonated eggs and inactivated with β‐propiolactone (Sigma Aldrich, St Louis, MO, USA). ELISA plates were coated with inactivated A/California/07/09 virus at 320 HA U/ml in PBS. Samples of sera were tested in a series of fourfold dilutions, and antigen‐specific antibodies were detected using a horseradish peroxidase‐conjugated goat anti‐mouse IgG antibody (Jackson Immunoresearch Laboratories Inc., West Grove, PA, USA).
For the HI assay, serum samples from immunized animals were first treated with a receptor‐destroying enzyme (RDE; Denka Seiken Co. Ltd., Tokyo, Japan). The HI assay was carried out using 0·75% turkey erythrocytes as described previously, 13 with an initial serum dilution of 1:20 against the A/Brisbane/59/07, A/Brisbane/10/07, B/Florida/4/06, A/California/07/09 × 179A, or A/Texas/5/09 × PR8 (NIBSC 07/346, 07/250, 07/248, CDC # 2009713114, or CDC #2009713119, respectively) viruses. Immunized animals with HI antibody titers ≥40 were defined as seropositive.
Results and Discussion
Production and characterization of plant‐produced HA antigens
Following the sequence optimization and cloning of HA antigens to obtain HAB1(H1) [A/Brisbane/59/07], HAB1(H3) [A/Brisbane/10/07], HAF1(B) [B/Florida/4/06], and HAC1[A/California/04/09], each HA molecule was produced in agroinfiltrated N. benthamiana plants using our launch vector system. Optimum levels of expression were observed at 7 DPI for all four HA antigens (data not shown). The total target protein levels were determined to range from 400–1300 mg/kg of fresh leaf tissue. As shown in Figure 2, HAB1(H1) and HAB1(H3) required the presence of Triton X‐100 (0·5%) for solubility, whereas HAF1(B) and HAC1 were soluble in the absence of the detergent.
Figure 2.
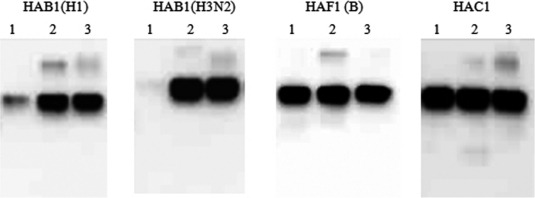
Expression and solubility profiles of hemagglutinin antigens produced in plants. Leaf tissue samples were collected at 7 DPI and homogenized in the extraction buffer without (total soluble protein: lane 1) or with 0·5% Triton X‐100 (total soluble protein with the detergent: lane 2), and with 1× gel loading buffer for total protein (lane 3). All samples were subjected to SDS–PAGE, and Western blot analysis was carried out using an anti‐6xHis mouse mAb.
All four HA antigens were purified from N. benthamiana plants using His‐tag‐based affinity chromatography. 10 As shown by SDS–PAGE analysis, the purity of the final preparations of the plant‐produced HA antigens was >85% (Figure 3).
Figure 3.
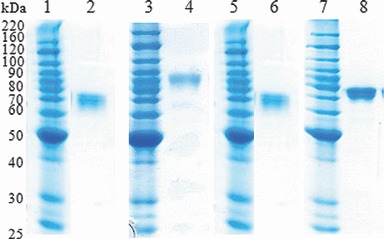
Purification of hemagglutinin (HA) antigens from plant tissue. Purified HA antigens were analyzed by SDS–PAGE followed by Coomassie staining. Lane 1, 3, 5, and 7: Molecular marker, lane 2: HAB1(H1), lane 4 HAB1(H3), lane 6: HAF1(B), and lane 8: HAC1.
Immunogenicity of plant‐produced HA antigens in mice
The immunogenicity of the plant‐produced HA antigens was individually evaluated in mice. Following immunization, samples of sera were collected, and both HA‐specific IgGs and HI antibody titers were measured. All animals immunized with HAB1(H1), HAB1(H3), or HAF1(B) showed serum HA‐specific IgG responses that increased after the antigen boosts (Figure 4). Of the animals immunized with a 60, 30, or 15 μg dose of HAB1(H1), 33·3%, 16·7%, and 33·3% of mice, respectively, had detectable serum HI antibodies after the first antigen boost, and these titers increased after the second antigen boost (Table 1 and Figure 5, left panel). After the second antigen boost, the proportions of seropositive animals in the groups that received a 15 or 30 μg dose of HAB1(H1) reached 100% (Table 1). Similarly, HI titers and seropositive rates increased after the second antigen boost among animals immunized with HAB1(H3) or HAF1(B) (Table 1 and Figure 5, middle and right panels).
Figure 4.
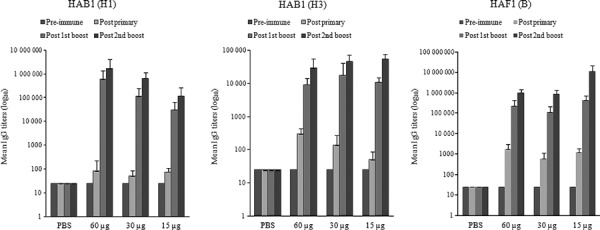
Titers of hemagglutinin (HA)‐specific IgG antibodies in mice immunized with HAB1(H1), HAB1(H3), or HAF1(B). Mice were immunized with a HA antigen three times, at days 0, 14, and 28, and serum samples were collected prior to each immunization and 2 weeks after the third dose (study day 42). Data are shown as the mean serum IgG titers ± SE.
Table 1.
Serum HI antibody responses against homologous virus elicited by seasonal influenza hemagglutinin antigens in mice
| GMT* | Seropositive rate (%)** | ||||
|---|---|---|---|---|---|
| Pre‐immune | Post‐primary | Post‐first boost | Post‐second boost | ||
| PBS/Quil A | 10 ± 0 | 10 ± 0 | 10 ± 0 | 10 ± 0 | 0/0 |
| HAB1(H1) | |||||
| 60 μg | 10 ± 0 | 10 ± 0 | 20·0 ± 24·5 | 71·3 ± 98·8 | 33·3/83·3 |
| 30 μg | 10 ± 0 | 10 ± 0 | 15·9 ± 11·5 | 100·8 ± 56·0 | 16·7/100 |
| 15 μg | 10 ± 0 | 10 ± 0 | 22·4 ± 24·1 | 113·1 ± 21·7 | 33·3/100 |
| PBS/Quil A | 10 ± 0 | 10 ± 0 | 10 ± 0 | 10 ± 0 | 0/0 |
| HAB1(H3) | |||||
| 60 μg | 10 ± 0 | 10 ± 0 | 25·2 ± 5·4 | 80·0 ± 45·1 | 50/83·3 |
| 30 μg | 10 ± 0 | 10 ± 0 | 56·6 ± 20·6 | 359·2 ± 96·3 | 83·3/100 |
| 15 μg | 10 ± 0 | 10 ± 0 | 50·4 ± 27·6 | 63·5 ± 25·5 | 66·7/83·3 |
| PBS/Quil A | 10 ± 0 | 10 ± 0 | 10 ± 0 | 10 ± 0 | 0/0 |
| HAF1(B) | |||||
| 60 μg | 10 ± 0 | 10 ± 0 | 25·2 ± 25·0 | 142·5 ± 133·7 | 33·3/83·3 |
| 30 μg | 10 ± 0 | 10 ± 0 | 28·3 ± 13·4 | 142·5 ± 89·1 | 50/100 |
| 15 μg | 10 ± 0 | 10 ± 0 | 14·1 ± 11·7 | 100·8 ± 42·7 | 16·7/83·3 |
HI, hemagglutination inhibition; PBS, phosphate‐buffered saline.
*GMT, geometric mean titer of HI antibodies ± SE.
**Post‐first boost/post‐second boost.
Figure 5.
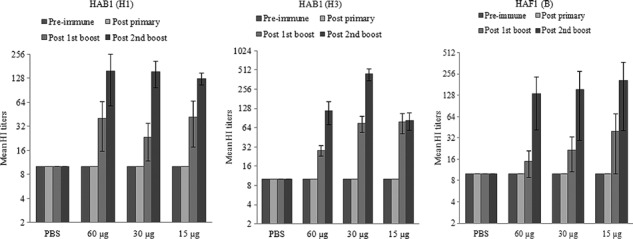
Titers of hemagglutination inhibition (HI) antibodies against seasonal influenza strains. Mice were immunized with HAB1(H1), HAB1(H3), or HAF1(B) three times, at days 0, 14, and 28, and serum samples were collected prior to each immunization and 2 weeks after the third dose (study day 42). Anti‐A/Brisbane/59/07, A/Brisbane/10/07, and B/Florida/4/06 HI antibody titers were expressed as the reciprocal of the highest dilution of serum that inhibits hemagglutination of eight hemagglutinin units of each virus. Samples without detectable HI antibody titers were assigned a titer of 10. Data shown are the mean titers (log2) from each group of immunized mice ± SE.
Evaluation of HAC1‐specific immune responses in mice immunized s.c. with HAC1 and Quil A adjuvant demonstrated that 100% of animals receiving a 30 μg dose and 83% of animals receiving a 15 or 5 μg dose of the antigen had serum IgG against A/California/07/09 after the primary immunization (data not shown), and the titers significantly increased after the antigen boost (Figure 6). Similar results were observed in mice immunized with a 30 μg dose of HAC1 absorbed to Alhydrogel and administered i.m. (Figure 6).
Figure 6.
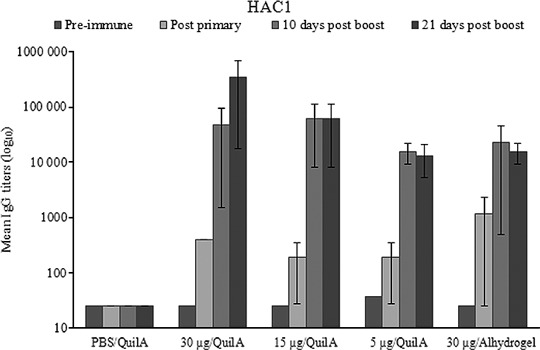
Titers of hemagglutinin‐specific IgG antibodies in mice immunized with HAC1. Mice were immunized with HAC1 twice, at days 0 and 21, and serum samples were collected prior to each immunization, as well as 10 and 21 days after the second dose (study days 31 and 42, respectively). The HAC1 antigen was administered to mice either in the presence of Quil A (s.c.) or adsorbed to Alhydrogel (i.m.). Anti‐A/California/07/09 IgG antibody titers were expressed as the mean serum IgG titers ± SE.
Serum HI antibody responses in mice immunized s.c. with HAC1 and Quil A were evaluated using A/California/07/09 or A/Texas/5/09, two novel H1N1 influenza A virus strains. Ten days after the antigen boost, all animals had very high serum HI antibody titers against A/California/07/09 (Figure 7, left panel). These titers slightly increased by 21 days after the antigen boost, reaching geometric mean titers [±standard errors (SE)] of 1:1810·2 ± 347·3, 1:1810·2 ± 373·3, or 1:806·3 ± 429·5 at a 30, 15, or 5 μg antigen dose, respectively (Table 2 and Figure 7, left panel). Animals that were immunized i.m. with a 30 μg/dose of HAC1 with Alhydrogel, which is the only adjuvant currently approved in the USA for human use, also showed substantial serum HI antibody production at 10 days after the antigen boost (Figure 7, left panel). Although the mean titer was lower compared to the mice immunized with HAC1 and Quil A, it was ≥1:40 in all animals at 10 days after the antigen boost and reached the level of 1:718·4 ± 350·5 at 21 days after the antigen boost (Table 2). Furthermore, all animals immunized with HAC1 in the presence of either Quil A or Alhydrogel had prominent HI antibody titers (>1:80) against A/Texas/5/09 (Figure 7, right panel).
Figure 7.
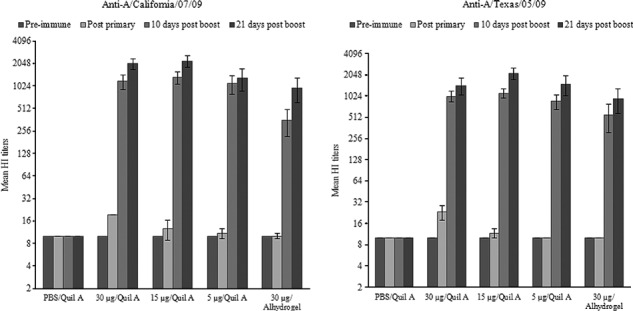
Titers of hemagglutination inhibition (HI) antibodies against the novel H1N1 influenza A strains. Mice were immunized with HAC1 twice, at days 0 and 21, and serum samples were collected prior to each immunization, as well as 10 and 21 days after the second dose (study days 31 and 42, respectively). The HAC1 antigen was administered to mice either in the presence of Quil A (s.c.) or adsorbed to Alhydrogel (i.m.). Anti‐A/California/07/09 and A/Texas/5/09 HI antibody titers were expressed as the reciprocal of the highest dilution of serum that inhibits hemagglutination of eight hemagglutinin units of each virus. Samples without detectable HI titers were assigned a titer of 10. Data shown are the mean titers (log2) from each group of immunized mice ± SE.
Table 2.
Serum HI antibody responses against A/California/07/09 elicited by HAC1 in mice
| GMT* | Seropositive rate (%)** | ||||
|---|---|---|---|---|---|
| Pre‐immune | Post‐primary | 10 days post‐boost | 21 days post‐boost | ||
| PBS/Quil A | 10 ± 0 | 10 ± 0 | 10 ± 0 | 10 ± 0 | 0/0/0 |
| HAC1 | |||||
| 30 μg/Quil A | 10 ± 0 | 17·3 ± 3·7 | 987·6 ± 269·8 | 1810·2 ± 347·3 | 0/100/100 |
| 15 μg/Quil A | 10 ± 0 | 12·0 ± 1·7 | 1220·1 ± 363·9 | 1810·2 ± 373·3 | 0/100/100 |
| 5 μg/Quil A | 10 ± 0 | 10·7 ± 0·8 | 712·2 ± 308·9 | 806·3 ± 429·5 | 0/100/100 |
| 30 μg/Alhydrogel | 10 ± 0 | 10 ± 0 | 232·8 ± 139·2 | 718·4 ± 350·5 | 0/83·3/100 |
HI, hemagglutination inhibition; PBS, phosphate‐buffered saline.
*GMT, geometric mean titer of HI antibodies ± SE.
**Post‐primary/10 days post‐boost/21 days post‐boost.
Taken together, the presented results demonstrate that the plant‐produced HA antigens from seasonal influenza viruses (HAB1[H1], HAB1[H3] and HAF1[B]) and a novel H1N1 influenza A virus (HAC1) induced serum HI antibody responses at the levels considered protective against these virus infections.
In summary, we engineered and produced recombinant HA antigens from seasonal influenza vaccine strains, A/Brisbane/59/07, A/Brisbane/10/07, and B/Florida/4/06, and from the novel H1N1 influenza A strain, A/California/04/09, in N. benthamiana plants using our launch vector system. As shown in Figure 1, these recombinant HAs were available as final vaccine antigens within 2 months of obtaining the amino acid sequences. Immunization of mice with these plant‐produced HA antigens induced serum IgG and HI antibody responses at the levels considered protective, showing the feasibility of our launch vector system for the rapid production of immunogenic vaccine antigens at a medium scale (1 kg of fresh biomass) in response to pandemic disease outbreaks, suggesting a potential for scale‐up. Recently, we have produced HAC1 in N. benthamiana plants at the large scale (50 kg of plant biomass) under cGMP conditions, purified it to the average yield of 90 mg/kg and demonstrated its safety and immunogenicity in preclinical animal models. 16 Currently, safety and immunogenicity of this plant‐produced H1N1 influenza vaccine candidate are being evaluated in a Phase 1 clinical trial. 17
Author contributions
Y. Shoji, C. E. Farrance, K. Musiychuk, B. J. Green, S. Sharma, J. A. Chichester, V. Mett and V. Yusibov conceived and designed the experiments. Y. Shoji, C. E. Farrance, J. Bautista, H. Bi, K. Musiychuk, A. Horsey, H. W. Park, J. Jaje, M. Shamloul and J. A. Chichester performed the experiments. Y. Shoji, C. E. Farrance, J. Bautista, K. Musiychuk, J. A. Chichester and V. Mett analyzed the data. Y. Shoji, C. E. Farrance, J. A. Chichester and V. Mett wrote the paper.
Acknowledgements
The authors thank Drs. Roger Hellens and Phil Mullineaux of the John Innes Centre for the pGreen binary vector system and Dr. Natasha Kushnir for editorial assistance.
References
- 1. Nichol KL, Treanor JJ. Vaccines for seasonal and pandemic influenza. J Infect Dis 2006; 194(Suppl 2):S111–S118. [DOI] [PubMed] [Google Scholar]
- 2. Lu B, Zhou H, Ye D, Kemble G, Jin H. Improvement of influenza A/Fujian/411/02 (H3N2) virus growth in embryonated chicken eggs by balancing the hemagglutinin and neuraminidase activities, using reverse genetics. J Virol 2005; 79:6763–6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bragstad K, Nielsen LP, Fomsgaard A. The evolution of human influenza A viruses from 1999 to 2006: a complete genome study. Virol J 2008; 5:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lugovtsev VY, Vodeiko GM, Strupczewski CM, Ye Z, Levandowski RA. Generation of the influenza B viruses with improved growth phenotype by substitution of specific amino acids of hemagglutinin. Virology 2007; 365:315–323. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention . Serum cross‐reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR Morb Mortal Wkly Rep 2009; 58:521–524. [PubMed] [Google Scholar]
- 6. The World Health Organization . Pandemic (H1N1) 2009‐update 112. Available at http://www.who.int/csr/don/2010_08_06/en/index.html (Accessed 10 August 2010). 2010.
- 7. Chichester JA, Haaheim LR, Yusibov V. Using plant cells as influenza vaccine substrates. Expert Rev Vaccines 2009; 8:493–498. [DOI] [PubMed] [Google Scholar]
- 8. Rybicki EP. Plant‐produced vaccines: promise and reality. Drug Discov Today 2009; 14:16–24. [DOI] [PubMed] [Google Scholar]
- 9. Chichester JA, Yusibov V. Plants as alternative systems for production of vaccines. Hum Vaccin 2007; 3:146–148. [DOI] [PubMed] [Google Scholar]
- 10. Mett V, Farrance CE, Green BJ, Yusibov V. Plants as biofactories. Biologicals 2008; 36:354–358. [DOI] [PubMed] [Google Scholar]
- 11. Musiychuk K, Stephenson N, Bi H et al. A launch vector for the production of vaccine antigens in plants. Influenza Other Respi Viruses 2007; 1:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shoji Y, Bi H, Musiychuk K et al. Plant‐derived hemagglutinin protects ferrets against challenge infection with the A/Indonesia/05/05 strain of avian influenza. Vaccine 2009; 27:1087–1092. [DOI] [PubMed] [Google Scholar]
- 13. Shoji Y, Chichester JA, Bi H et al. Plant‐expressed HA as a seasonal influenza vaccine candidate. Vaccine 2008; 26:2930–2934. [DOI] [PubMed] [Google Scholar]
- 14. Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM. pGreen: a versatile and flexible binary Ti vector for Agrobacterium‐mediated plant transformation. Plant Mol Biol 2000; 42:819–832. [DOI] [PubMed] [Google Scholar]
- 15. Green BJ, Fujiki M, Mett V et al. Transient protein expression in three Pisum sativum (green pea) varieties. Biotechnol J 2009; 4:230–237. [DOI] [PubMed] [Google Scholar]
- 16. Shoji Y, Chichester JA, Jones M et al. Plant‐based rapid production of recombinant subunit hemagglutinin vaccines targeting H1N1 and H5N1 influenza. Hum Vaccin 2011; 7(Suppl):41–50. [DOI] [PubMed] [Google Scholar]
- 17. Fraunhofer USA Center for Molecular Biotechnology . Fraunhofer USA CMB Plant Produced H1N1 Vaccine Begins Phase 1 Clinical Trial. Available at http://www.businesswire.com/news/home/20100917006118/en/Fraunhofer‐USA‐CMB‐Plant‐Produced‐H1N1‐Vaccine (Accessed 17 September 2010).


