Abstract
Please cite this paper as: Balasegaram et al. (2012) Patterns of early transmission of pandemic influenza in London – link with deprivation. Influenza and Other Respiratory Viruses 6(3), e35–e41.
Background During the early containment phase in England from April to June 2009, the national strategy for H1N1 pandemic influenza involved case investigation and treatment, and tracing and prophylaxis of contacts.
Objective To describe the relationship between early transmission of H1N1 pandemic influenza in London and age and socio‐economic status.
Methods Epidemiological data on cases of pandemic flu in London reported to the London Flu Response Centre were analysed to determine patterns of transmission.
Results There were 3487 reported cases (2202 confirmed, 1272 presumed and 14 probable) from 20 April to 28 June 2009, during the ‘containment’ period. The highest report rate of 206 per 100 000 (95% CI 195–218) was seen in primary school–age children (5−11 years) followed by 129 (95% CI 119–139) in secondary school–age children (12–18 years). Reports of cases were initially concentrated in affluent areas but overall showed a clear trend with deprivation and risk ratio of 2·32 (95% CI 1·94–2·78) between the most deprived and the least deprived.
Conclusion Early transmissions were highest amongst school‐aged children but linked with socio‐economic deprivation across all age groups.
Keywords: influenza, pandemic, socio‐economic deprivation
Introduction
In the UK, the national containment strategy for H1N1 pandemic influenza involved case investigation and treatment, and tracing and prophylaxis of contacts, from 25 April to 2 July 2009. 1 , 2 , 3
In London, information on detailed epidemiology was collected on cases from the initial case on 27 April to the onset of widespread community transmission (week of June 29). 4 , 5
Modelling of early transmission patterns from the UK suggests school‐based transmission accounting for 37% of cases, based on 60% of cases with epidemiologically determined likely source of infection. 3 , 6 In using data from a single city, the pattern of early transmission of influenza leading up to established community spread can be described.
Methods
Cases were referred by attending clinicians from both hospital and community clinics and investigated by the London Flu Response Centre (FRC) based on symptoms, history of travel to an affected area and/or contact with a confirmed or probable case. 1 , 2 Exact definitions used for investigation were those in the national algorithms that were published online and frequently updated with changes. In addition, individuals who were tested by other routes (primary care physicians, influenza surveillance schemes) and whose results were positive were referred to the FRC by the reporting laboratories.
Data on cases and contacts were obtained from the FRC database developed for the pandemic (Fluzone, Infact UK Ltd., Yorkshire, UK). Cases were assigned to calendar weeks according to the date of onset of their symptoms, so for the purpose of this paper, the containment period includes only cases with symptom onset within the period Monday 20 April–Sunday 28 June 2009 (calendar weeks 17–26).
Data on laboratory results were obtained from data sets sent to the London FRC from four laboratories in London that performed specific testing. Initially, local laboratories tested for influenza A, with positive samples sent to the national reference laboratory for specific pandemic assays. From 4 June 2009 (week 23), pandemic specific H1 and N1 assays became available in these four London laboratories. 1 The laboratory data set included all results on pandemic specific assays tested regardless of the source of the test. Hence, by cross‐referencing the laboratory data set with the FRC database, individuals who were tested by other routes (primary care physicians, influenza surveillance schemes) and whose results were positive could be investigated and added to the FRC database.
Case definitions are given in Box 1. To investigate transmission patterns, cases were categorised on initial report by the epidemiologically likely source of infection, using the following national definitions, as typing was not available at this time:
Table Box 1.
Case definitions for H1N1 pandemic influenza, England (April 2009) 2
| Possible case: a person with a fever or self‐reported history of fever (38°C) and symptoms of an acute respiratory illness and recent travel to an affected area, or contact with a confirmed or probable case |
| Probable case: a person who was a possible case and tested positive for influenza A that was non‐subtypable |
| Confirmed case: a person who tested positive for pandemic H1N1 2009 influenza virus by specific real‐time reverse transcriptase polymerase chain reaction (RT‐PCR) confirmed by sequence analysis |
| Presumed case: a person with a clinical diagnosis (acute respiratory symptoms) without laboratory confirmation but with an epidemiological link to a previously confirmed case. This case definition was introduced at the end of May 2009 |
-
1
Imported: history of travel to an affected area (IMexico, and initially specified areas of the United States, subsequently all of the United States) within 7 days of onset.
-
2
School related (secondary, tertiary or presumed): contact within 7 days of onset with a symptomatic case in a school.
-
3
Secondary or tertiary (non‐school related): contact within 7 days of onset with a symptomatic travel associated or secondary case in a non‐school setting.
-
4
Presumed (non‐school related): a presumed case with an epidemiological link to a non‐school‐related case.
-
5
Community: no travel in the 7 days before onset and no contact with a confirmed case or setting (or possible case with symptoms) within 7 days of onset.
-
6
Unassigned: insufficient information available to categorise into one of the above categories.
To investigate the link with deprivation, cases were assigned to Lower Layer Super Output Areas (LSOAs) by postcode of residence, which were in turn mapped to Index of Multiple Deprivation (IMD) 2007 quintiles. LSOAs are geographical areas, which are used to report small area statistics in England and Wales. They are built from clusters of adjacent unit postcodes and have a mean population of 1500. 7 The IMD is a national index that combines a number of indicators, chosen to cover a range of economic, social and housing issues, into a single deprivation score for each small area in England. The IMD is produced at LSOA level, with each LSOA allocated to a quintile, based on its national relative level of deprivation, with quintile 1 being the most deprived and quintile 5 the least deprived. 8
The number of confirmed and presumed cases reported to the FRC per 100 000 population was calculated using mid‐year 2008 age‐specific population figures for the London Government Office region, and report by level of deprivation was calculated using mid‐year 2008 LSOA population estimates (2009 estimates were not available), to give the report of cases per 100 000 population in each deprivation quintile. 9 Reports on the most deprived (IMD quintile 1) LSOAs were compared with reports on the two least deprived LSOAs (IMD quintile 4 and 5) by calculating risk ratios (RR), including 95% confidence intervals (CI) and P‐values, using Stata 10·1. IMD (Stata Corp LP., College Station, TX, USA) quintiles 4 and 5 were combined to give comparable population sizes (total population of IMD1 LSOAs was 2 234 992, compared with 1 709 262 for IMD 4 and five LSOAs combined). Maps showing location of cases were produced using Map Info. Cases with missing or invalid postcodes (668, 19% of the total cases) were excluded from the deprivation analysis and the maps.
Results
Demographics
A total of 3487 cases were reported between 20 April and 28 June 2009, of which 2202 (63%) were confirmed cases, 1272 (36%) were presumed cases, and 13 (0·4%) were probable cases. Of the 3202 cases where sex was reported, 1666 (52%) were male.
The overall London report risk was 46 per 100 000 population (95% CI 44–47). The highest report of 206 per 100 000 (95% CI 195–218) was seen amongst the primary school age group of 5‐ to 11‐year‐olds, followed by 129 (95% CI 119–139) in the secondary school age group of 12‐ to 17‐year‐olds; 45 (95% CI 40–51) in preschool children aged four or younger and 22 (95% CI 21–23) in adults aged 18 or older. When presumed and possible cases were removed from the data analysis, the same overall pattern was seen, with the highest reporting of confirmed cases seen in children aged 5–11 (131), followed by children aged 12–17 (91) and 0–4 (22) and adults aged 18 and over (14 per 100 000).
Information about hospitalisation was recorded for 3478 cases (99·7%). Forty cases were hospitalised (1%), with eight of these reported as having an underlying medical condition. Eight cases were documented as being pregnant, five of whom (63%) were hospitalised. Four deaths were recorded (0·1%), in which two individuals (aged 8 and 39) had an underlying medical condition, another was pregnant (aged 39), and the fourth death was in an elderly person (aged 80).
The number of individuals tested and new cases (positivity) per week initially increased slowly up to week 24, when there was a marked rise at the start of week 25 (Figure 1, Table 1). These data are an underestimate of the true number of individuals tested as peripheral laboratories would not have reported those individuals testing negative for influenza A. From week 22, the proportion of tests that are positive for pandemic H1 rises up to week 26.
Figure 1.
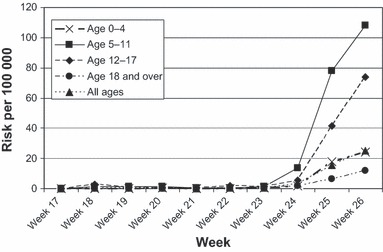
Age specific risk for school age groups.
Table 1.
Positivity risk by week
| Week | Flu A | H1 | ||
|---|---|---|---|---|
| Tests carried out | Positivity (%) | Tests carried out | Positivity (%) | |
| 17 | 5 | 20 | 3 | 33 |
| 18 | 169 | 6 | 7 | 29 |
| 19 | 290 | 10 | 23 | 0 |
| 20 | 219 | 11 | 12 | 0 |
| 21 | 144 | 6 | 14 | 21 |
| 22 | 101 | 13 | 88 | 15 |
| 23 | 242 | 17 | 223 | 17 |
| 24 | 349 | 17 | 318 | 19 |
| 25 | 1422 | 31 | 1458 | 32 |
| 26 | 1592 | 44 | 3330 | 45 |
| Total | 4533 | 29 | 5476 | 38 |
The testing was first available only in the national reference laboratory until it was rolled out subsequently into the local London laboratories in week 23. In the early weeks, only samples that first tested positive for A were then sent to the national laboratory for pH1N1 testing. Once the local laboratories had set up the pH1N1 test, they could then run both assays simultaneously.
Reports amongst primary‐ and secondary‐school‐aged children remained consistently higher than in other age groups (Figure 1). This pattern may partly reflect the emphasis on surveillance in schools during the early weeks of the pandemic.
Transmission patterns
Information on likely method of transmission was available for 1554 (98%) of the 1581 presumed and confirmed cases in weeks 17–25. Transmission patterns changed over weeks 17–24, until week 25 when most cases were acquired through school transmission (Figure 2). By week 26, infection was so widespread in the community that there was no longer value in categorising cases by likely source of infection.
Figure 2.
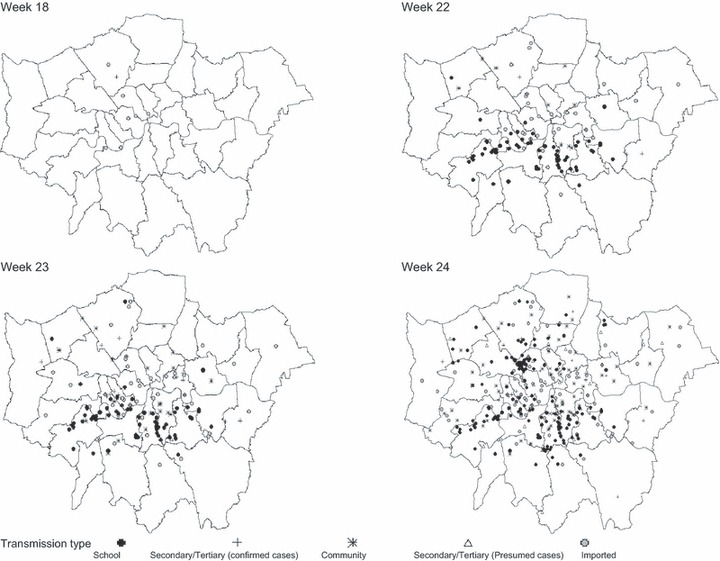
Geographical spread of cases in London by time and transmission route.
In week 17, there were five imported cases and one secondary case, all in affluent parts of London. Week 18 was marked by the onset of school‐related outbreaks in south London, accounting for 24 of 26 cases in this week.
School‐related transmission accounted for 58% of cases (72 of 124) from weeks 17–22, with 31 (22%) of cases imported, 11 (9%) thought to be due to community transmission and 7 (6%) acquired through non‐school‐related secondary transmission (usually household related, Figure 3). After school closure for half‐term in week 23, there were 103 new school–related cases (45%) in week 24, but the proportion of community cases (22%) remained similar to the previous week. In week 25, school outbreaks contributed to a total of 330 (28%) school‐related cases, by then spread across London.
Figure 3.
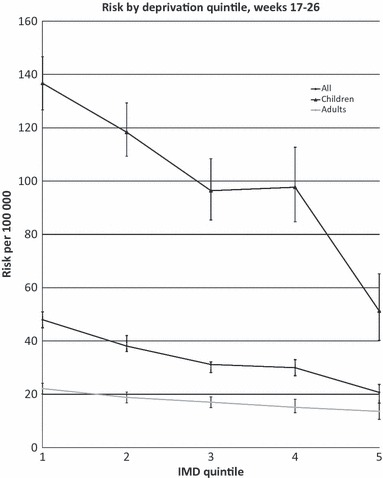
Cumulative risk by deprivation quintile.
In total, 110 cases reported a history of travel outside the UK in the 7 days prior to onset of illness, of which 92 (6% of total cases) were considered to have imported infection. School‐related transmission accounted for 506 cases (32%), with other transmission (household or other close contact) accounting for 458 (29%).
Community transmission accounted for 498 (25%) of all cases with the majority (426 cases) in week 25.
Relationship with deprivation
Eighty‐one per cent of cases (2819) had valid postcodes, with the proportion of valid postcodes being over 94% up to and including week 24, falling to 85% and 75% in weeks 25 and 26, respectively.
Analysis of all cases from week 17–26 showed a consistent trend of a higher risk in the more deprived populations (Figure 3). For all ages, the risk was 48 per 100 000 (95% CI 45–51), 38 (95% CI 36–42), 31 (95% CI 28–34), 30 (95% CI 36–42) and 21 (95% CI 17–24), for IMD 1 (most deprived), IMD 2, IMD 3, IMD 4 and IMD 5 (least deprived), respectively. The risk ratio from IMD 1 to IMD 5 was 2·32 (95% CI 1·94–2·78, P = 0·001).
The results were consistent across age and gender and when only confirmed cases were analysed. The effect was marked in children (under 16 years); the risk in IMD 1 was 137 (95% CI 127–147) and fell to 51 (95% CI 40–65) in IMD 5; the risk ratio IMD 1/IMD 5 was 2·67 (95% CI 2·06–3·49, P < 0·001). In adults, the corresponding risks were 22 (95% CI 20–24) to 14 (95% CI 11–17) with a risk ratio IMD 1/IMD 5 of 1·63 (95% CI 1·27–2·12, P = 0·001).
As there were only a few cases and no significant differences in risk in the initial weeks, cases from weeks 17–23 were grouped together and averaged to give a mean weekly risk (Figure 4). The mean weekly risk ratio up to the end of week 23 between the most deprived (IMD quintile 1) and the least deprived (quintiles 4 and 5 combined) was 0·67 (95% CI 0·43‐ 1·04 2 sided P = 0·07). However, the risk ratio widened to 1·7 (95% CI 1·12–2·44, P = 0·011) in week 24, 1·9 (95% CI 1·56–2·27, P < 0·001) in week 25 and 2·1 (95% CI 1·80 ‐2·47, P < 0·001) in week 26.
Figure 4.
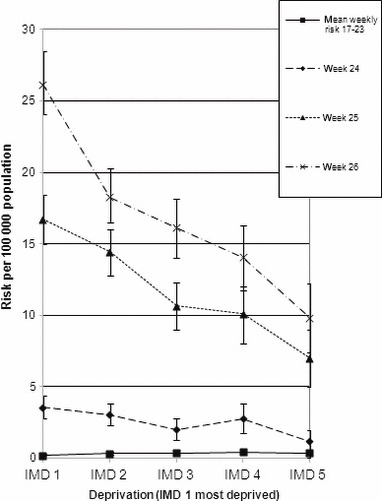
Risk of cases in each Index of Multiple Deprivation quintile by week.
In the period of week 17–23, the cumulative risk ratio between the most deprived and the least deprived was 0·52 (95% CI 0·33–0·80, P = 0·003). In contrast, the cumulative risk ratio up to the end of week 26 was 1·82 (95% CI 1·63–2·03 P < 0000).
Discussion
This study describes the initial spread of pandemic influenza in a major city to the point when community transmission was widespread. In the first seven weeks, there was little change in risk. The average relative risk of 0·67 (CI 0·43–1·04) between the most deprived and least deprived populations from week 17–23 suggests that there is a non‐significant possibility of higher transmission in the affluent groups or at least no increased risk in the deprived group. Subsequently, once infection starts to spread in week 24–26, initial spread was related to school transmissions and was consistently higher in the deprived population.
Our data comprised individuals who tested positive for pandemic H1N1 influenza in London at the time and is thus a reflection of those seeking health care. The key bias was the criteria for investigation set out in the national algorithms. Although there were frequent changes in the algorithms up to 3 June 2009, the criteria were reasonably consistent in their emphasis on fever of 38°C, travel to affected areas or contact with a known case.
The cases that would not be picked up were those that did not fit these criteria – those with mild infection without fever were not recognised or treated during the early weeks. 1 Some of these cases were identified in London from week 19 by other routine testing systems such as the routine influenza syndromic testing or by investigations initiated by primary and secondary care services. 10
To correct this discrepancy, from 3 June 2009 (week 23), the algorithms were widened to pick up individuals who did not meet the original criteria. This change in policy predates the sudden increase in cases and tests by almost two weeks; hence, it is unlikely that the prevailing investigating criteria masked any major increase in severe cases.
Epidemiological data on transmission patterns are subject to misclassification. For example, the number of cases classified as ‘community’ transmissions in school‐aged children, where insufficient information was available to define school transmission, may have resulted in an underestimation of the number of school‐related cases. This is likely to be a bigger effect than the number of children classified as school related who may have acquired infection by another route. Hence, it is likely that misclassification would underestimate the effect of spread in schools. In addition, cases in children may be overestimated in relation to other age groups as parents may be more likely to seek health care for their children than for other age groups.
With these limitations, deprivation and school‐based transmission play a key role in the early transmission of pandemic influenza. The link with material deprivation has been observed with data on hospitalisations and infectious respiratory illness, and outpatient and emergency department visits for seasonal flu. 11 , 12 Our study reflects individuals seeking health care; thus, any biases in health‐seeking behaviours with primary or acute care would be reflected. Data on access to antiviral prescribing centres during the pandemic in another area in the UK suggest that more deprived communities have a reduced use of antiviral centres. 13 Thus, any similar biases in health‐seeking behaviour in London would tend to underestimate the effect of deprivation in our study; yet, the higher risk with deprivation persists. This effect is consistent with syndromic surveillance data, showing that the peak in London was in week 29, with earlier peaks in one of the most deprived boroughs in week 27. 10 The pattern of higher risk with deprivation was similar to the West Midlands and may reflect overcrowding (a factor in the IMD deprivation score), more mixing and larger household size. 14 , 15 , 16 However, as our study only describes the early phase of transmission, we cannot infer end rates of acquisition in different socio‐economic groups.
Our data are also consistent with the high attack rates in school‐aged children that have been reported in previous pandemics and in the H1N1 pandemic both in London and elsewhere and in population serological studies in the UK conducted in August and September 2009, showing the highest seroconversion rates to be in children in London and the West Midlands. 17 , 18 , 19 , 20 , 21
Community transmission in London took eight weeks to become widespread, which may have been moderated by the natural closure of schools for half‐term and/or the public health interventions of widespread prophylaxis, exclusion of symptomatic cases and proactive school closure; however, any intervention effect is likely to have been minor. 2
In conclusion, the initial transmission pattern of H1N1 pandemic influenza in London shows the earlier spread to the wider community to be associated with transmission amongst school‐aged children and marked an association with deprivation.
.
Funding
The authors have no sources of financial funding.
Competing Interests
The authors have no competing interests.
Ethical approval
Not required.
Acknowledgements
We would like to thank all the staff who worked in the London Flu Response Centre for their work in collecting the data, with special thanks to Rachel Strudwick for help with initial data cleaning. We would also like to thank staff who assisted from the HPA London Regional Epidemiology Unit, especially Neelam Alhaddad for help with data cleaning and analysis and Stephen McKenzie for producing the maps used in this study.
References
- 1. Health Protection Agency . Pandemic H1N1 2009 in England: an overview of initial epidemiological findings and implications for the second wave. Available at http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1258560552857 (Accessed 25 May 2011).
- 2. Cabinet Office . An independent review of the UK response to the 2009 influenza pandemic, 1 July 2010. Available at http://interim.cabinetoffice.gov.uk/media/416533/the2009influenzapandemic‐review.pdf (Accessed 25 May 2011).
- 3. McLean E, Pebody RG, Campbell C et al. Pandemic (H1N1) 2009 influenza in the UK: clinical and epidemiological findings from the first few hundred (FF100) cases. Epidemiol Infect 2010; 138:1531–1541. [DOI] [PubMed] [Google Scholar]
- 4. Balasegaram S, Glasswell A, Cleary V, Turbitt D, McCloskey Bl. From containment to community: trigger points from the London pandemic (H1N1) 2009 influenza incident response. Public Health 2011; 125:272–278. [DOI] [PubMed] [Google Scholar]
- 5. Cleary V, Balasegaram S, Keeling D, McCloskey B, Turbitt D. Pandemic (H1N1) 2009: setting up a multi‐agency regional response centre: a tool kit for other public health emergencies. J Bus Contin Emer Plan 2010; 4:154–164. [PubMed] [Google Scholar]
- 6. Ghani A, Baguelin M, Friffin J et al. The Early transmission dynamics of H1N1 pdm influenza in the United Kingdom. PLoS Curr 2009; 1:RRN1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. NHS Data Model and Dictionary Service . NHS business definitions: lower layer super output area. Available at http://www.datadictionary.nhs.uk/data_dictionary/nhs_business_definitions/l/lower_layer_super_output_area_de.asp?shownav=1 (Accessed 25 May 2011).
- 8. Communities and Local Government . Indices of deprivation 2007. Available at http://www.communities.gov.uk/publications/communities/indiciesdeprivation07 (Accessed 25 May 2011).
- 9. Office for National Statistics . Super output area mid‐year population estimates for England and Wales (experimental). Available at http://www.statistics.gov.uk/StatBase/Product.asp?vlnk=14357&More=Y (Accessed 25 May 2011).
- 10. Smith S, Smith GE, Olowokure B et al. Early spread of the 2009 influenza A(H1N1) pandemic in the United Kingdom – use of local syndromic data, May‐August 2009. Eurosurveillance 2011; 16:pii 19771. [PubMed] [Google Scholar]
- 11. Hawker JL, Olowokure B, Sufi F et al. Social deprivation and hospital admission for respiratory infection: an ecological study. Respir Med 2003; 97:1219–1224. [DOI] [PubMed] [Google Scholar]
- 12. Charland KM, Brownstein JS, Verma A, Brien S, Buckeridge DL. Socio‐economic disparities in the burden of seasonal influenza: the effect of social and material deprivation on rates of influenza infection. PLoS One 2011; 6:e17207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haroon S, Barbosa G, Saunders P. The determinants of health‐seeking behaviour during the A/H1N1 influenza pandemic: an ecological study. J Public Health 2011; 33:503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Health Protection Agency West Midlands H1N1v Investigation Team . Preliminary descriptive epidemiology of a large school outbreak of influenza A (H1N1) in the West Midlands, United Kingdom May 2009. Eurosurveillance 2009; 14:pii 19264. [DOI] [PubMed] [Google Scholar]
- 15. Semanza J, Giesecke J. Intervening to reduce inequalities in infectious diseases in Europe. Am J Public Health 2008; 98:787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cardoso MRA, Cousesens SN, Siqueira LF, Alves FA, D’Angelo LA. Crowding: risk factor or protective factor for lower respiratory disease in young children? BMC Public Health 2004; 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jordan WS, Denny FW, Badger GF et al. A study of illness in a group of Cleveland families. XVII. The occurrence of Asian Influenza. Am J Hyg 1958; 68:190–212. [DOI] [PubMed] [Google Scholar]
- 18. Monto A, Koopman J, Longini I. The Tecumseh study of illness XIII. Influenza infection and disease, 1076–1981. Am J Epidemiol 1985; 121:811–822. [DOI] [PubMed] [Google Scholar]
- 19. Fox JP, Hall CE, Cooney MK et al. Influenza virus infections in Seattle families 1975‐1979. II Pattern of infection in invaded households and relation of age and prior antibody to occurrence of infection and related illness. Am J Epidemiol 1982; 116:228–242. [DOI] [PubMed] [Google Scholar]
- 20. Woodall J, Rowson KEK, McDonald J. Age and Asian influenza 1957. Br Med J 1957; 1:1316–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross sectional study. Lancet 2010; 375:1100–1108. [DOI] [PubMed] [Google Scholar]


