Abstract
The present paper is a continuation of a series of comprehensive taxonomic treatments of cercosporoid fungi (formerly Cercospora s. lat.), belonging to Mycosphaerellaceae (Ascomycota). This fifth contribution of this series proceeds with treatments of cercosporoid fungi on dicots and comprises species occurring on hosts belonging to the families Anacardiaceae and Annonaceae, which are described and illustrated in alphabetical order under the particular cercosporoid genera, supplemented by keys to the species concerned. A detailed introduction, a survey of currently recognised cercosporoid genera, a key to the genera concerned, and a discussion of taxonomically relevant characters were published in the first part of this series. The following taxonomic novelties are introduced: Passalora cotini sp. nov., P. guoana nom. nov., P. rhois-aromaticae sp. nov., and Pseudocercospora rhoicola.
Keywords: Ascomycota, Cercosporas. lat., hyphomycetes, taxonomy
INTRODUCTION
One of the largest groups of fungi belonging to Mycosphaerellaceae (Capnodiales, Ascomycota) includes genera and species akin to the genus Cercospora which are commonly referred to as cercosporoid fungi (Crous & Braun 2003, Braun et al. 2013). The taxa concerned comprise dematiaceous, holoblastic asexual morphs and at least partly mycosphaerella-like sexual morphs. Corresponding mucedinaceous fungi within Mycosphaerellaceae are often classified as ramularioid fungi. Ramularia, the eponymous genus, is associated with sexual morphs belonging to Mycosphaeraella s. str. The type species of the latter genus has a phylogenetically proven asexual morph pertaining to Ramularia (Verkley et al. 2004) making Mycosphaerella a younger heterotypic synonym of Ramularia, which has been proposed as the preferred name (Crous et al. 2009c, Wijayawardene et al. 2014, Videira et al. 2015) and recommended for protection (Rossman et al. 2015).
Cercosporoid fungi represent a very large group of plant pathogenic, leaf-spotting, economically relevant species causing diseases on a wide range of hosts, including numerous cultivated plants. In the last few decades, enormous taxonomic progress has been made by increasing application of molecular methods in capnodialean fungi, including Mycosphaerellaceae (Crous et al. 2013, Groenewald et al. 2013, Bakhshi et al. 2014, Nguanhom et al. 2015, Guatimosim et al. 2016, etc.). Cultures and molecular sequence analyses should be used for taxonomic purposes in this fungal group whenever possible. On the other hand, symptoms and morphological characters exhibited in vivo are still important in diagnosing plant pathogenic fungi, especially for routine monitoring and identification purposes, but also in light of the ecology and taxonomy of the fungi concerned, which cause diseases on living plants. Therefore, optimal descriptions of new and already known species should cover the full range of traits ranging from characters in vivo, in vitro to phylogenetic data. Currently only a limited number of cercosporoid fungi has been cultivated and sequenced, and hundreds of taxa are only known from characters in vivo. However, all names have to be taken into consideration for taxonomic purposes and are subject to the rules of the International Code of Nomenclature for algae, fungi, and plants (ICN; McNeill et al. 2012), including Art. 11 dealing with the priority of names. Thus, all old names are applicable when dealing with taxonomic-nomenclatural questions, i.e. to find correct names for certain taxa in accordance with the Code. To facilitate this process, careful examinations of all old type collections and detailed descriptions are necessary and helpful for sound taxonomic conclusions. This is precisely the sense of this series, based on Chupp’s (1954) old, seriously outdated, Cercospora monograph, and meant to represent an updated, supplementary treatment intended as a platform for the routine identification of cercosporoid fungi, including modern molecular examinations. We have spent decades examining thousands of old type collections and other samples of cercosporoid fungi, inter alia in preparation for the annotated list of Cercospora and Passalora names published by Crous & Braun (2003). Numerous previously unpublished results have been incorporated in already published issues of the present series. Cultures, sequence analyses and epitypifications on the basis of cultured and sequenced collections represent the next phase in order to transfer the old morphology-based names into the era of consolidated species concepts.
The growing number of DNA sequences of cercosporoid fungi allows better insights in the phylogenetic structure of this fungal group on the familial as well as the generic level. On the other hand, it has gradually been pointed out that traditionally applied phenotypic traits of the sexual as well as asexual morphs of cercosporoid fungi (Mycosphaerellaceae) are not in full agreement with genotypic generic circumscriptions. Species only forming mycosphaerella-like sexual morphs, i.e. without any asexual fructifications, are known in many currently recognised cercosporoid genera (e.g. Guatimosim et al. 2016). As a consequence, the generic affinity of mycosphaerelloid ascomycetes cannot be properly resolved without cultures, sequences, and phylogenetic analyses. On the other hand, the classical morphological characters of asexual morphs of cercosporoid fungi are no longer reliable for allocating certain species to particular genera. The analysis of fern-inhabiting cercospoid fungi recently examined in Brazil (Guatimosim et al. 2016) is a striking example. The genus Zasmidium is known to be polyphyletic (Crous et al. 2009a, b, Braun et al. 2013). Besides genuine species of Zasmidium s. str., zasmidium-like asexual morphs are also known for the cercosporoid genera Neoceratosperma (Crous et al. 2014, Guatimosim et al. 2016) and Paramycosphaerella (widened to include species with zasmidium-like asexual morphs by reallocations of Zasmidium aerohyalosporium, Z. dicranopteridis, Z. nabiacense, and Mycosphaerella parkii (i.e. Z. parkii; Guatimosim et al. 2016), i.e. cercosporoid species with zasmidium-like morphology cannot be properly assigned to cercosporoid genera without data from sequence analyses. The situation in all other cercosporoid genera is comparable. Pseudocercospora-like phenotypes of cercosporoid asexual morphs are usually connected with the genus Pseudocercospora s. str., but there are exceptions. Several species morphologically not or barely distinguishable from true Pseudocercospora species do not cluster within the large Pseudocercospora clade and have been placed in Pallidocercospora and Phaeocercospora (Crous et al. 2012, 2013, Braun et al. 2013). Phaeophloeospora pteridivora is an additional species with a pseudocercosporoid asexual morph that clusters in a clade with coelomycetous morphs (Guatimosim et al. 2016). The cercospora-like morphology of conidiophores and conidia was believed to be a reliable character to assign species to Cercospora s. str., but even in this genus there are exceptions, for example the recently introduced genus Neocercospora (Bakhshi et al. 2015). However, the crux of the matter within Cercospora s. str. is that reliable identifications at species level are barely possible without multigene analyses, above all within the Cercospora apii s. lat. complex (Groenewald et al. 2013). Passalora s. lat. (sensu Crous & Braun 2003, Braun et al. 2013) is another strongly polyphyletic genus in which the morphological characters of the asexual morphs traditionally used for the separation of genera do not reflect phylogenetic entities (i.e. genera). A corresponding detailed phylogenetic treatment of the Passalora complex is under preparation. All results of modern studies dealing with cercosporoid fungi in Mycosphaerellaceae show that proper taxonomic results and conclusions have to be based on an integrated approach including molecular sequence analyses. Conclusions just based on morphological analyses are unreliable and do not allow proper generic allocations. Therefore, we have decided to revise the focus of the present series. Efforts to reassess and reallocate cercosporoid genera and species will thus in future be based on re-examinations of types and other collections in combination with cultures and sequence analyses whenever possible. The present series will not be continued in its present form. In future, it will rather focus on the revision of type collections of old cercosporoid species, including comprehensive descriptions and illustrations, which are necessary as basis for modern examinations in vitro including subsequent sequence analyses.
So far four contributions have been published: part one dealing with cercosporoid fungi on other fungi (mycophylic taxa), on ferns as well as gymnosperms (Braun et al. 2013); part two dedicated to species on monocots, excluding true grasses (Braun et al. 2014); part three with a treatment of cercosporoids on hosts of Poaceae (Braun et al. 2015a); and part four as the first contribution to cercosporoids on dicots covering species occurring on hosts belonging to the families Acanthaceae to Amaranthaceae (Braun et al. 2015b). Part five is a continuation covering cercosporoid fungi on hosts of the dicot families Anacardiaceae and Annonaceae. Generic descriptions and keys to accepted genera are included in the first part. The structure of the present part follows the principles circumscribed in part 1 (Braun et al. 2013).
MATERIALS AND METHODS
The present work is a compilation based on our previous papers and unpublished data, as well as global literature. Details of methods are given in the papers cited under References. As far as new examinations are concerned, fungal structures have been studied by standard methods of light microscopy, using an Olympus BX50 microscope, with distilled water and lactic acid as mountants, but without any stain. If possible, measurements of 30 conidia and other structures have been made at a magnification of ×1000. All illustrations have been prepared by UB. The following abbreviations are used: author names follow Brummit & Powell (1992), journals Bridson (2004a, b), and exsiccatae http://www.botanischestaatssammlung.de/DatabaseClient/IndExs/index.jsp (IndExs – Index of Exsiccatae). Taxonomy and nomenclature of plant families, genera and species are based on the “Angiosperm Phylogeny Website” (http://www.mobot.org/mobot/research/apweb/), Tropicos database (http://www.tropicos.org/), and The Plant List (http://www.theplantlist.org).
TAXONOMIC TREATMENT
Cercosporoid species on dicots s. lat. (Anacardiaceae to Annonaceae)
Anacardiaceae
Cercospora
Key to Cercospora species on Anacardiaceae
1 External hyphae with solitary conidiophores present in vivo; conidia solitary or occasionally catenate, obclavate, base obconically truncate, to about 140 μm long and 4–7.5 μm wide; on Rhus semialata .............................................................................................................................................. see Passalora guoana
Mycelium consistently internal in vivo; solitary conidiophores not developed; conidia consistently solitary, acicular, base truncate, 2–6 μm wide ...................................................................................................................... 2
2 (1) Leaf spots finally greyish brown to dingy greyish white with brown border; on Mangifera indica ............................................................................................................................................... C. mangiferae-indicae
Leaf spots yellowish brown to blackish brown; on Rhus, Spondias, and Toxicodendron spp. ............................................................................................................................................................ C. verniciferae
Tabular key to Cercospora species on Anacardiaceae according to host genera
Mangifera
A single species ....................................................................................................................................... C. mangiferae-indicae
Rhus
1 External hyphae with solitary conidiophores present in vivo; conidia solitary or occasionally catenate, obclavate, base obconically truncate, to about 140 μm long and 4–7.5 μm wide see .............................................................................................................................................. Passalora guoana
Mycelium consistently internal in vivo; solitary conidiophores not developed; conidia consistently solitary, acicular, base truncate, 2–5 μm wide .................................. Cercospora verniciferae
Spondias
A single species ....................................................................................................................................................C. verniciferae
Toxicodendron
A single species .................................................................................................................................................... C. verniciferae
Cercospora species on Anacardiaceae
Cercospora mangiferae-indicae Munjal, Lall & Chona, Indian Phytopathol. 14: 185 (1962) [“1961”].
(Fig. 1)
Fig. 1.
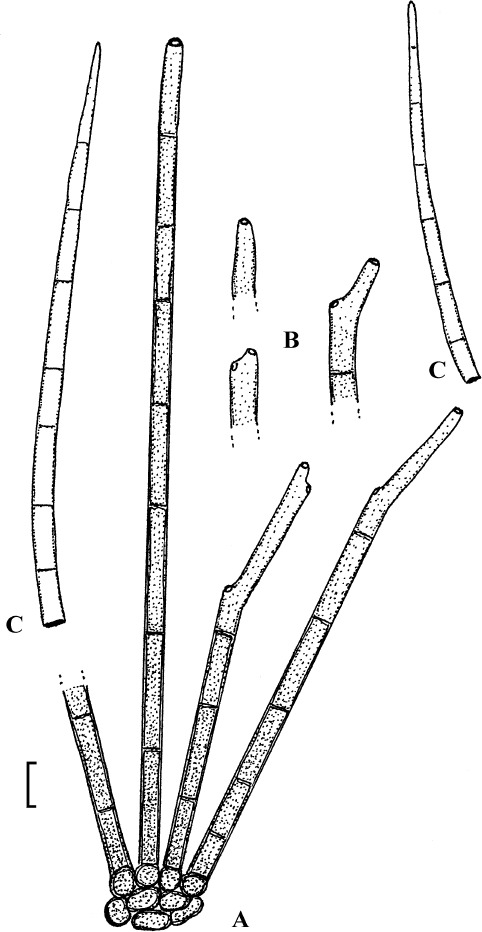
Cercospora mangiferae-indicae (BPI 438068A). A. Conidiophore fascicle. B. Conidiophore tips. C. Conidia. Bar = 10 μm.
Literature: Crous & Braun (2003: 266), Kakoti et al. (1998), Kamal (2010: 62), Todawat Nawalsing & Papdiwal (2011).
Illustration: Munjal et al. (1962: 187, fig. 6).
Description: Leaf spots amphigenous, subcircular to irregular, 1–25 mm diam, scattered to gregarious, pale to medium dark brown, later with greyish brown to dingy greyish white centre and brown border on the upper leaf surface, brown with darker border below. Caespituli amphigenous, but mainly epiphyllous, scattered, delicately punctiform, dark brown. Mycelium internal. Stromata lacking or small, 10–30 μm diam, substomatal or immersed, intraepidermal, only composed of a few swollen hyphal cells, 3–10 μm diam, brown, wall somewhat thickened. Conidiophores in small to moderately large fascicles, mostly 2–20, erect, straight, subcylindrical or attenuated towards the tip, unbranched, not to moderately geniculate, 15–250 × 3–8 μm, pluriseptate, pale to medium brown or olivaceous brown throughout or paler towards the tip, wall slighty thickened, smooth; conidiogenous cells integrated, terminal, sometimes intercalary, 15–40 μm long, conidiogenous loci conspicuous, thickened and darkened, 2–4 μm diam. Conidia solitary, acicular, straight to curved, 20–200 × 3–6 μm, 1- to pluriseptate, hyaline, thin-walled, smooth, apex subacute, base truncate, 3–4.5 μm wide, hila thickened and darkened.
Holotype: India: New Delhi, India Agricultural Research Institute, on Mangifera indica, 11 Dec. 1959, V. Prakash (HCIO 26847).
Host range and distribution: On Mangifera indica, Anacardiaceae, Asia (India, Asom, Delhi, Maharashtra, Tamil Nadu), West Indies (Dominican Republic).
Notes: This is a true Cercospora s. str., morphologically belonging to C. apii s. lat., but with relatively broad conidia, 3–6 μm. Records from the Dominican Republic refer to collections deposited as BPI 438068A,B. Conidiophores and conidia are morphologically barely distinguishable from those of C. verniciferae. The phylogenetic affiliation of this species is unknown.
Cercospora verniciferae Chupp & Viégas, Bol. Soc. Brasil. Agron. 8: 56 (1945).
(Fig. 2)
Fig. 2.
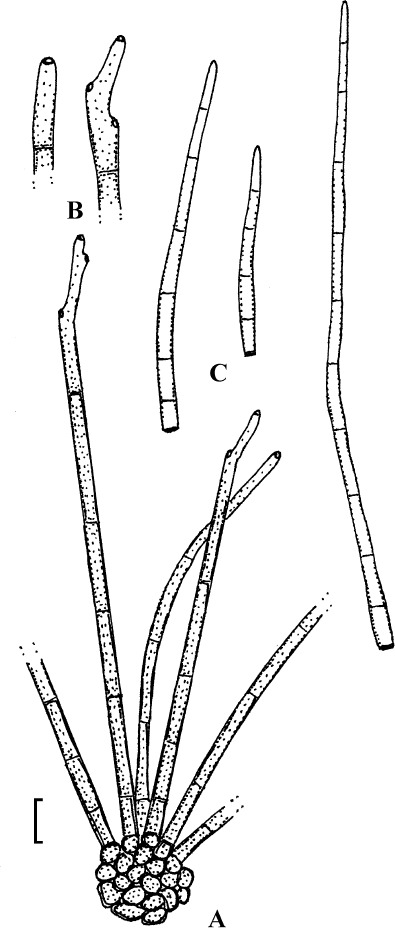
Cercospora verniciferae (CUP 41508, lectotype). A. Conidiophore fascicle. B. Conidiophore tips. C. Conidia. Bar = 10 μm.
Literature: Chupp (1954: 44), Crous & Braun (2003: 420), Guo et al. (2005: 32–33), Phengsintham et al. (2012: 50–51; 2013: 98).
Illustration: Guo et al. (2005: 33, fig. 14), Phengsintham et al. (2012: 52, fig. 1a, 53, fig. 1b; 2013: 99, figs 44–45).
Description: Leaf spots amphigenous, subcircular to angular-irregular, 1.5–10 mm diam, later confluent and larger, yellowish brown to blackish brown. Caespituli hypophyllous. Mycelium internal. Stromata absent or small, substomatal, composed of a few dark brown cells. Conidiophores in small to moderately large fascicles, 2–20, arising from internal hyphae or stromatic hyphal aggregations, through stomata, divergent, erect, straight, subcylindrical to somewhat geniculate, unbranched, 25–345 × 3–5.5 μm, pluriseptate, medium to dark brown throughout or paler towards the tip, wall thin to somewhat thickened, smooth; conidiogenous cells integrated, terminal and intercalary, conidiogenous loci conspicuous, 1.5–3 μm diam. Conidia solitary, acicular, straight to curved or sinuous, 30–175 × 2–5 μm, 3- to pluriseptate, hyaline, thin-walled, smooth, apex subacute, base truncate, 1.5–3 μm wide, thickened and darkened.
Lectotype (designated here, MycoBank, MBT204884): Brazil: São Paulo: Campinas, Santa Elisa Farm, Instituto Agronomico, on Toxicodendron verniciferum [Rhus vernicifera], Anacardiaceae, 28 Apr. 1936, A. S. Costa 1518 (CUP 41508). Isolectotype: IACM 1518.
Host range and distribution: On Rhus (chinensis var. roxbourgii, succedanea), Spondias (dulcis, pinnata), Toxicodendron verniciferum, Anacardiaceae, Asia (Thailand), Oceania (Samoa), South America (Brazil).
Notes: This species belongs to the Cercospora apii s. lat. complex. The identity of morphologically barely distinguishable collections on Spondias species from Asia and Oceania, referred to as C. verniciferae, is unclear and need molecular confirmation. The phylogenetic affiliation of C. verniciferae and its relation to C. mangiferae-indicae are unknown.
Doubtful, excluded and insufficiently known species
Cercospora anacardii-occidentale Rangasw. et al., Scheme for Collection & Identification of Fungi of South India, Final Report: 12 (1969); nom. inval. (Art. 39.1).
Literature: Crous & Braun (2003: 56), Kamal (2010: 16).
Note: Cercospora anacardii-occidentale, described from south India on Anacardium occidentale is an invalid name. Type material could not be traced, and the taxonomic status of this species is unclear.
Cercospora megaspermae L.N. Bhardwaj & R.C. Sharma, Indian Forester 120: 545 (1994).
Literature: Kamal (2010: 64).
Illustration: Bharadwaj & Sharma (1994: 546, fig. 1a).
Description: Leaf spots small, irregular, 1–5 mm diam. Mycelium internal. Conidiophores fasciculate, unbranched, 75–180 × 5–15 μm, septate, brown to dark brown. Conidia solitary, narrowly obclavate-linear, straight to curved, 60–172 × 2–4 μm, 3–8-septate, subhyaline to pale olivaceous.
Holotype: India: Himachal Pradesh: Solan, on Pistacia integerrima, Anacardiaceae, 15 Oct. 1989, L. N. Bharadwaj (Mycol. Herb., Dept. of Mycology & Pl. Pathol., Dr. Y.S. Pamar Univ. of Horticulture & Forestry, Solan, India, MH 515).
Host range and distribution: Only known from the type collection.
Notes: Type material of this species was not available for examination. The original description is very meagre, and the conidia were described as subhyaline to pale olivaceous, and details of the conidiogenous loci were not given. The identity of this insufficiently described species is unclear.
Cercosporella toxicodendri (Ellis) U. Braun, Monogr. Cercosporella, Ramularia and Allied Genera (Phytopath. Hyphom.) 2: 402 (1998).
Basionym: Cercospora toxicodendri Ellis, Amer. Naturalist 16: 811 (1882).
Synonyms: Pseudocercospora toxicodendri (Ellis) X.J. Liu & Y.L. Guo, Mycosystema 2: 237 (1989).
Mycovellosiella toxicodendri (Ellis) U. Braun, Mycotaxon 55: 224 (1995).
Cercosporella californica Bonar, Mycologia 34: 188 (1942) [holotype: USA: California: San Mateo County, on Toxicodendron diversilobum, 15 Aug. 1935, L. Bonar (UC 653836); isotypes: BPI 420516, FH, MICH 15390, WIS-F-11579].
Literature: Saccardo (1886: 467), Chupp (1954: 43), Guo & Hsieh (1995: 14), Braun (1995a: 68; 1998: 402).
Illustrations: Braun (1995: 69, fig. 66, as C. californica).
Exsiccatae: Barthol., Fungi Columb. 4708. Ellis & Everh., Fungi Columb. 691. Ellis & Everh., N. Amer. Fungi 1524, 1524b.
Description: Leaf spots amphigenous, scattered, subcircular to irregular, 1–6 mm diam, sometimes confluent and larger, at first pale, greyish, brownish, later dark brown to almost black, surrounded by a darker margin. Caespituli hypophyllous, punctiform, whitish. Mycelium immersed; hyphae hyaline, septate, sparingly branched, forming loose stromatic hyphal aggregations, substomatal, subhyaline, composed of swollen hyphal cells, 3–5 μm diam. Conidiophores few to numerous, in dense fascicles, arising from internal hyphae or stromata, through stomata, erect, straight and subcylindrical to moderately geniculate-sinuous, unbranched, 10–35 × (3–)4–7(–8.5) μm, 0–1-septate, hyaline, thin-walled, smooth, often somewhat attenuated towards the tip; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, 10–25 μm long, conidiogenous loci convex, slightly thickened, refractive. Conidia solitary, obclavate-fusiform to subcylindrical, 40–70(–90) × 3–4 μm, (0–)2–6(–8)-septate, hyaline, thin-walled, smooth, apex subobtuse to subacute, base short obconically truncate, hila slightly thickened, refractive.
Neotype (designated by Braun 1998: 401): USA: New Jersey: Gloucester County, Newfield, on Toxicodendron pubescens, Sep. 1885, J. B. Ellis [Ellis & Everh., N. Amer. Fungi 1524] (NY 838637). Isoneotypes: Ellis & Everh., N. Amer. Fungi 1524, e.g., BPI 441967, 441971, CUP 41424, NY 838639–838641, ILL 98792, OSC 35014, PAD, RMS 3281, WIS-F-9652.
Host range and distribution: On Rhus (venenata, Rhus sp.), Toxicodendron (diversilobum [Rhus diversiloba], orientale [Rhus ambigua], pubescens [Rhus toxicodendron], radicans subsp. radicans, radicans subsp. rydbergii [Rhus rydbergii]), Anacardiaceae, North America (Canada; USA, Florida, Indiana, Iowa, Massachusetts, Maryland, New Jersey, North Carolina, Texas).
Notes: The complicated nomenclature and taxonomy of this species has been discussed by Braun (1998). Type material of C. toxicodendri, collected in Newfield before or in 1882, is not preserved at NY and could also not be traced in any other herbaria, but topotype material collected in 1885 is preserved in many herbaria. This material, designated by Braun (1998) as neotype, contains two fungi, one with colourless conidiophores and conidia and a second with pigmented structures, conspecific with Cercospora bartholomei. Ellis (1882) described colourless conidia agreeing with the colourless Cercosporella present in the neotype material. Therefore, Braun (1998) restricted the name C. toxicodendri to the Cercosporella element and neotypified this species accordingly. The correct name for Cercospora toxocodendri sensu Chupp (1954) is Passalora bartholomei.
Passalora (s. lat.)
Key to Passalora species on Anacardiaceae
1 Superficial hyphae with solitary conidiophores formed in vivo ........................................................................................ 2
Superficial hyphae with solitary conidiophores not formed in vivo .................................................................................. 5
2 (1) Conidia solitary, at most rarely in short chains; stromata lacking or small, 10–20 μm diam ........................................... 3
Conidia often or at least partly in chains; stromata larger, 15–90 μm diam .................................................................... 4
3 (2) Conidia subhyaline to pale olivaceous brown, 2.5–5 μm wide; on Rhus and Toxicodendron spp., North America ................................................................................................................................... P. bartholomei
Conidia hyaline, 4–6.5 μm wide; on Rhus sp., China ....................................................................................... P. guoana
4 (2) Conidiophores 4–5 μm wide; conidia (0–)1–7-septate; on Rhus chinensis var. roxburghii, Asia ........................ P. rhoina
Conidiophores 2–4 μm wide; conidia 0–4(–6)-septate; on Myracrodruon urundeuva, South America .................................................................................................................................................... P. myracrodruonis
5 (1) Conidia obclavate-cylindrical, 4–11.5 μm wide, consistently solitary or at most in short chains ..................................... 6
Conidia narrower, 3–5 μm, ± cylindrical and in chains or obclavate-cylindrical and consistently solitary ....................... 7
6 (5) Conidia 15–135 × 4–11.5 μm, 0–10-septate, solitary or in short chains; on Sesaria parviflora, India ......................................................................................................................................... P. pithoragarhensis
Conidia 20–65 × 4–7 μm, 0–4-septate, consistently solitary; on Cotinus coggygria, USA ................................... P. cotini
7 (5) Conidia formed singly, obclavate-cylindrical; on Rhus aromatica, USA ........................................... P. rhois-aromaticae
Conidia formed in chains, ± cylindrical ............................................................................................................................. 8
8 (7) Conidia (10–)15–40(–50) μm long, 0–1(–3)-septate ................................................................................... P. marmorata
Conidia 20–120 μm long, 0–7-septate .................................................................................................................. P. rhois
Tabular key to Passalora species on Anacardiaceae according to host genera
Cotinus
A single species ................................................................................................................................................................ P. cotini
Myracrodruon
A single species ............................................................................................................................................. P. myracrodruonis
Rhus s. lat. (incl. Searsia and Toxicodendron)
1 Superficial hyphae with solitary conidiophores formed in vivo ......................................................................................... 2
Superficial hyphae with solitary conidiophores not formed in vivo .................................................................................. 4
2 (1) Conidia at least partly in chains; stromata large, 15–90 μm diam ..................................................................... P. rhoina
Conidia solitary, at most rarely in short chains; stromata lacking or small, 10–20 μm diam ........................................... 3
3 (2) Conidia subhyaline to pale olivaceous brown, 2.5–5 μm wide; on Rhus and Toxicodendron spp., North America ................................................................................................................................... P. bartholomei
Conidia hyaline, 4–6.5 μm wide; on Rhus sp., China .......................................................................................P. guoana
4 (1) Conidia obclavate-cylindrical, 15–135 × 4–11.5 μm; on Sesaria parviflora, India ............................ P. pithoragarhensis
Conidia narrower, 3–5 μm wide; on Rhus and Searsia spp. ............................................................................................ 5
5 (4) Conidia formed singly, obclavate-cylindrical; on Rhus aromatica, USA ........................................... P. rhois-aromaticae
Conidia formed in chains, ± cylindrical ............................................................................................................................. 6
6 (5) Conidia (10–)15–40(–50) μm long, 0–1(–3)-septate ................................................................................... P. marmorata
Conidia 20–120 μm long, 0–7-septate .................................................................................................................. P. rhois
Passalora species on Anacardiaceae
Passalora bartholomei (Ellis & Kellerm.) U. Braun & Crous, Mycosphaerella Anam. 1: 77 (2003).
(Fig. 3)
Fig. 3.
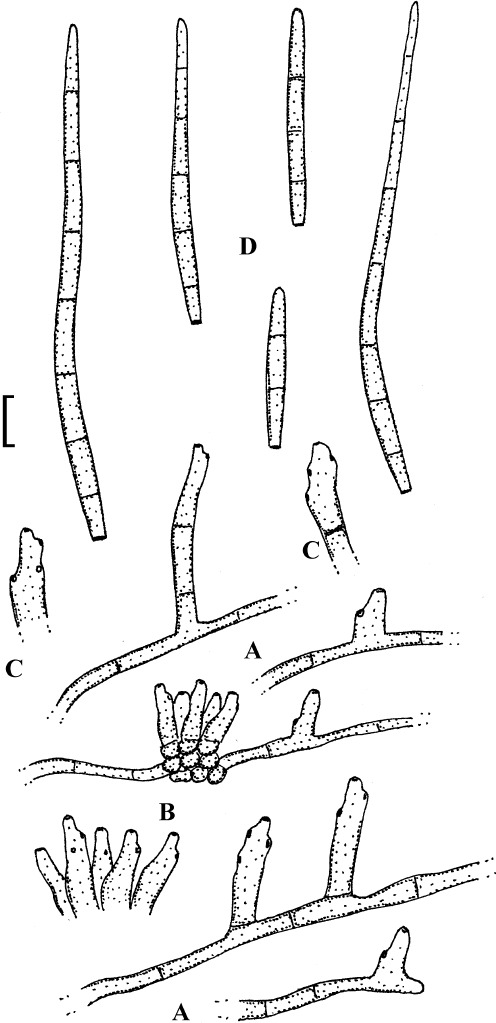
Passalora bartholomei (NY 830171, lectotype). A. Solitary conidiophores arising from superficial hyphae. B. Conidiophore fascicles. C. Conidiophore tips. D. Conidia. Bar = 10 μm.
Basionym: Cercospora bartholomei Ellis & Kellerm., J. Mycol. 5: 144 (1889).
Synonym: Mycovellosiella bartholomei (Ellis & Kellerm.) U. Braun, Monogr. Cercosporella, Ramularia Allied Genera (Phytopath. Hyphom.) 2: 402 (1998).
Literature: Saccardo (1892: 639), Chupp (1954: 43), Braun (1995b: 224; 1998: 402).
Illustration: Braun (1995b: 229, fig. 4, as Mycovelosiella toxicodendri).
Exsiccatae: Barthol., Fungi Columb. 3206. Barthol., N. Amer. Fungi 2980. Ellis & Everh., Fungi Columb. 696. Kellerman & Swingle, Kansas Fungi 1290.
Description: Leaf spots small, 1–5 mm diam, indistinct to angular-irregular, greenish to brown. Caespituli hypophyllous, punctiform to effuse, brownish. Mycelium internal and external; superficial hyphae emerging through stomata, branched, septate, 1.5–4 μm wide, olivaceous to brown, thin-walled, smooth. Stromata absent or small, 10–20 μm diam, brown. Conidiophores in small to moderately large fascicles, loose to fairly dense, arising from internal hyphae or stromata, through stomata or erumpent, or conidiophores solitary, arising from superficial hyphae, erect, straight, subcylindrical-conical to geniculate-sinuous, unbranched, 10–50 × 3–6 μm, 0–3-septate, olivaceous brown to brown, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, 5–25 μm long, conidiogenous loci conspicuous, thickened and darkened, 1.5–2 μm diam. Conidia solitary, obclavate-cylindrical, straight to curved or even sigmoid, (20–)25–160(–225) × 2.5–5 μm, 2–12-septate, subhyaline to pale olivaceous brown, thin-walled, smooth, apex obtuse to subacute, base short obconically truncate, 1.5–2 μm wide, hila slightly thickened and darkened.
Lectotype (designated here, MycoBank, MBT204885): USA: Kansas: Rooks County, on Toxicodendron pubescens [Rhus toxicodendron], 22 Sep. 1888, E. Bartholomew (NY 830171). Isolectotype: BPI 433259.
Host range and distribution: On Rhus glabra, Toxicodendron (pubescens, radicans subsp. radicans, radicans subsp. rydbergii [Rhus rydbergii]), Anacardiaceae, North America (Canada; USA, Indiana, Kansas, Wisconsin).
Note: Several names have been misapplied to this species, in particular that of Cercospora toxicodendri Ellis (Braun, 1998).
Passalora cotini U. Braun, sp. nov.
MycoBank MB816979
(Fig. 4)
Fig. 4.
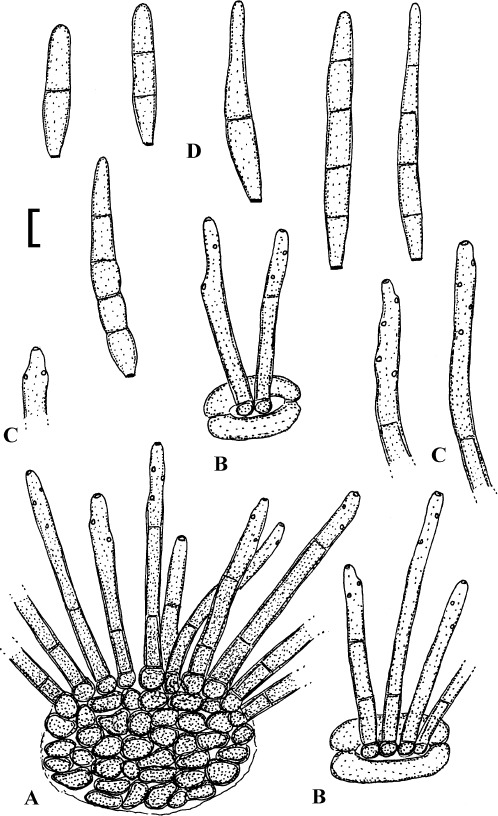
Passalora cotini (BPI 440737, holotype). A. Conidiophore fascicle arising from an immature ascoma. B. Conidiophore fascicles. C. Conidiophore tips. D. Conidia. Bar = 10 μm.
Etymology: Named after the host genus, Cotinus.
Diagnosis: Differs from Passalora pithoragarhensis in having shorter and narrower consistently solitary conidia, 20–65 × 4–7 μm, 0–4-septate.
Holotype: USA: Alabama: Tuskegee, on Cotinus coggygria, Anacardiaceae, 7 Aug. 1932, G. W. Carver (BPI 440737).
Description: Leaf spots amphigenous, subcircular to angular-irregular, 1–8 mm diam, or confluent and larger, forming large patches, ochraceous, pale to medium brown, margin indefinite or centre later pale, dingy greyish brown, grey to greyish white, with darker border. Caespituli amphigenous, punctiform, dark brown to blackish, scattered to gregarious. Mycelium internal. Stromata lacking or only formed as small substomatal aggregation of a few swollen hyphal cells, 10–20 μm diam, medium to dark brown, in addition with larger immersed stromata, 25–80 μm diam, medium to dark brown, cells 3–8 μm diam, wall thickened, which probably represent immature ascomatal initials. Conidiophores in small divergent fascicles (2–8), arising from internal hyphae or small substomatal hyphal aggregations, through stomata, and in small to large fascicles (to about 20), arising from larger stromata (ascomatal initials), loose to dense, erect, straight to curved, subcylindrical, slightly attenuated towards the tip or often subclavate (width somewhat enlaged from base to top), not to slightly geniculate-sinuous, unbranched, 20–80 × 3–8 μm, aseptate or with 1–3 thin, not very conspicuous septa, medium to medium dark brown throughout or paler towards the tip, wall somewhat thickened, above all in the lower half, to 1 μm wide, smooth; conidiogenous cells integrated, terminal, 10–40 μm long, or conidiophores aseptate, i.e. reduced to conidiogenous cells, with a single to mostly several, sometimes numerous conidiogenous loci, conspicuous, thickened and darkened, 1–2 μm diam. Conidia solitary, obclavate-cylindrical, straight to somewhat curved, 20–65 × 4–7 μm, (0–)1–4-septate, subhyaline to pale olivaceous or olivaceous brown, thin-walled, smooth, apex obtuse, broadly rounded to subacute, base short obconically truncate, 1.5–2 μm wide, hila somewhat thickened and darkened.
Host range and distribution: Only known from the type collection.
Notes: Type material of this species was originally deposited as Cercospora rhoina. Other cercosporoid collections on Cotinus coggygria belong to Pseudocercospora cotini. The new species on Cotinus belongs to Passalora s. lat., based on the broad morphological concept of that genus outlined in Crous & Braun (2003) and Braun et al. (2013). It is a species with Passlora s. str./Cercosporidium-like morphology, i.e. with consistently internal mycelium in vivo and conidia consistently formed singly. However, Passalora s. lat. is polyphyletic. A phylogenetic revision of this complex is under preparation. Since phylogenetically circumscribed genera and traditionally applied morphological concepts within Passalora s. lat. are not in accordance with each other, phylogenetically correct reallocations of particular species are in future only possible on the base of molecular data. Hence, the new species on Cotinus can currently (tentatively) only be assigned to Passalora s. lat.
Passalora guoana U. Braun, nom. nov.
MycoBank MB816980
(Fig. 5)
Fig. 5.
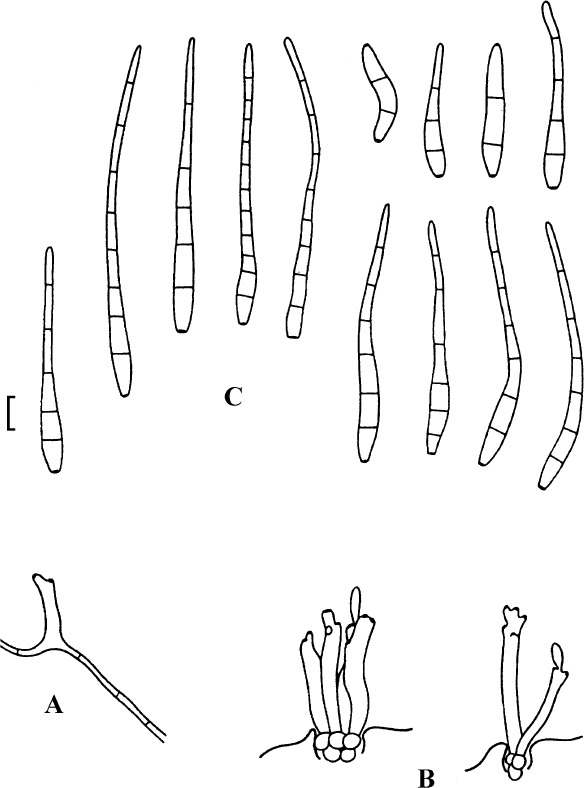
Passalora guoana (based on Guo & Jiang 2000: 264, fig. 4)). A. Superficial hypha with solitary conidiophore. B. Conidiophore fascicles, C. Conidia. Bar = 10 μm.
Basionym: Mycovellosiella rhois Y.L. Guo, in Guo & Jiang, Mycotaxon 74: 264 (2000); as “rhoidis”, nom. illegit. (Art. 53.1), non Mycovellosiella rhois Goh & W.H. Hsieh 1987.
Synonym: Passalora rhois (Y.L. Guo) Y.L. Guo, Mycosystema 30: 867 (2011); as “rhoidis”, nom. illegit. (Art. 53.1), non Passalora rhois (E. Castell.) U. Braun & Crous 2003.
Etymology: Named after the Chinese mycologist Y.L. Guo.
Literature: Guo et al. (2003: 12–14).
Illustration: Guo & Jiang (2000: 264, fig. 4), Guo et al. (2003: 13, fig. 2).
Description: Leaf spots amphigenous, subcircular to angular-irregular, 1–4 mm diam, often confluent, centre brown to dark brown, margin blackish brown on the upper side, grey to pale greyish brown below. Caespituli hypophyllous. Mycelium internal and external; superficial hyphae emerging through stomata or arising from conidiophore tips, branched, 1.7–3.2 μm wide, septate, subhyaline, thin-walled, smooth. Stromata absent. Conidiophores in small fascicles, 2–8, loose to dense, emerging through stomata, or solitary, arising from superficial hyphae, erect, straight to slightly curved or somewhat geniculate-sinuous, unbranched, irregular in width, 13–65 × 4–7.5 μm, 0–1-septate, hyaline to very pale olivaceous, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, conidiogenous loci conspicuous, thickened and darkened, 1.7–2.2 μm diam. Conidia solitary, occasionally in short chains, obclavate, straight to curved, 26–138 × 4–6.5 μm, 2–11-septate, hyaline, thin-walled, smooth, apex obtuse to subacute, base short obconically truncate, hila thickened and darkened.
Holotype: China: Hubei Province: Shennongjia, on Rhus sp., Anacardiaceae, 2 Aug. 1984, Y. L. Guo 212 (HMAS 77433).
Host range and distribution: Only known from the type collection.
Notes: The generic affinity of this species is quite unclear and not yet phylogenetically proven. Owing to colourless conidia, this species could even be allied to Cercospora s. str. However, Passalora is polyphyletic, and as long as the true generic affinity of this fungus remains unknown, we prefer to retain this species in Passalora s. lat. Mycovellosiella rhois Y.L. Guo as well as Passalora rhois (Y.L. Guo) Y.L. Guo are illegitimate names. Therefore, a new legitimate name is required for this species. The epithet “rhoidis” is undoudedly the wrongly formed genitive of Rhus that must correctly be “rhois”, and cannot be interpreted as an arbitrarily formed name. Furthermore, “rhoidis” and “rhois” (the wrong and correct genitive of Rhus) would be confusable names according to Art. 53.3 that have to be treated as homonyms.
Passalora marmorata (Tranzschel ex Sacc.) U. Braun & Crous, Mycosphaerella Anam. 1: 267 (2003).
(Fig. 6)
Fig. 6.
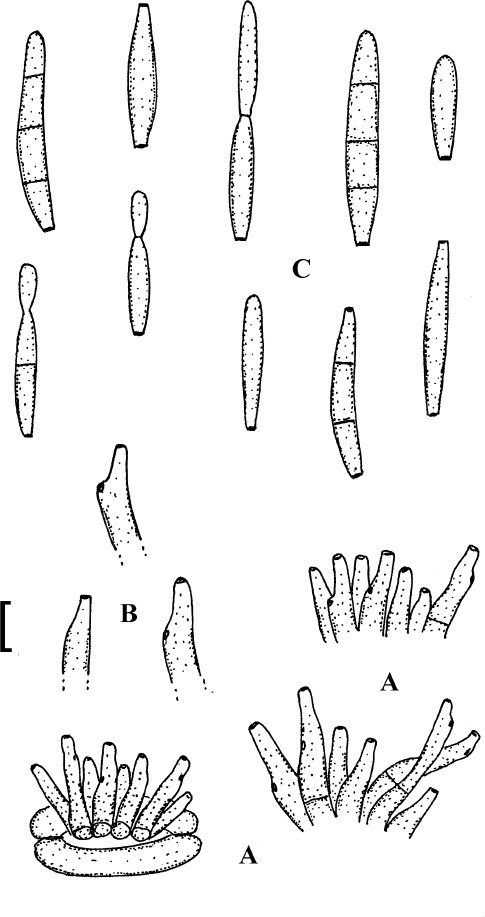
Passalora marmorata (LE 40410, lectotype). A. Conidiophore fascicles. B. Conidiophores. C. Conidia. Bar = 10 μm.
Basionym: Cercosporina marmorata Tranzschel ex Sacc., Syll. Fung. 25: 895 (1931); as “(Tranzschel) Sacc.”
Synonyms: Cercospora marmorata Tranzschel, in Tranzschel & Serebrianikow, Mycotheca Rossica, Fasc. 5, no. 250 (1911); nom. nud.
Phaeoramularia marmorata (Tranzschel ex Sacc.) Deighton, Mycol. Pap. 144: 34 (1979).
Cladosporium rhois Arcang., in Thümen, Mycoth. Univ., no. 1371 (1879), non Passalora rhois (E. Castell.) U. Braun & Crous 2003 [lectotype (designated here, MycoBank, MBT204886): Italy: Firenze, Settignano, on Rhus coriaria, Nov. 1879, G. Arcangeli [Thüm., Mycoth. Univ. 1371] (HAL). Isolectotypes: Thüm., Mycoth. Univ. 1371 (e.g. BPI 427440, HBG, K, PAD, S), and Baglietto et al., Erb. Crit. Ital., Ser. II, 849 (e.g. E, FH, G, NY).
Cercospora rhois-coriariae Kuhnh.-Lord., Ann. Épiphyt., ser. 2, 13: 54 (1947) [type: France: Département Var, on Rhus coriaria, M. Kuhnholtz-Lordat, not traced, probably not preserved].
Literature: Chupp (1954: 40), Ellis (1976: 316), Deighton (1979: 34), Braun & Mel’nik (1997: 72), Crous & Braun (2003: 267.
Illustrations: Ellis (1976: 316, fig. 239 A), Deighton (1979: 35, fig. 18).
Exsiccatae: Baglietto et al., Erb. Critt. Ital., ser. II, 849. Roum., Fungi. Sel. Exs. 4990. Thüm., Mycoth. Univ. 1371. Tranz. & Serebr., Mycoth. Ross. 250.
Description: Leaf spots amphigenous, angular-irregular, often vein-limited, 3–6 mm diam, sometimes to 10 mm diam, pale brown to ochraceous-grey, sometimes concentrically zonate with narrow brown lines and margin. Caespituli amphigenous, often hypophyllous, punctiform, dark brown. Mycelium internal; hyphae branched, septate, 2–4 μm wide, pale olivaceous, thin-walled, smooth. Stromata small, mostly substomatal, 10–30 μm diam, olivaceous brown. Conidiophores in small to moderately large fascicles (to about 50), divergent to dense, arising from internal hyphae or stromata, usually through stomata, erect, straight, subcylindrical to somewhat narrowed towards the tip, somewhat geniculate-sinuous, unbranched, 10–40 × 3–5 μm, 0–1-septate, pale to moderately olivaceous to yellowish brown, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores often reduced to conidiogenous cells, 10–25 μm long, conidiogenous loci conspicuous, thickened and darkened, 1–2 μm diam. Conidia in short to moderately long chains, occasionally in branched chains, cylindrical, subcylindrical, ellipsoid-fusiform, one-celled conidia sometimes obovoid, straight to curved, (10–)15–40(–50) × (2.5–)3–5(–5.5) μm, 0–1(–3)-septate, subhyaline to olivaceous, thin-walled, smooth, ends rounded to short obconically truncate, 1.5–2 μm wide, hila somewhat thickened and darkened.
Lectotype (designated by Braun & Mel’nik 1997: 72): Russia: “prope Simeis, Tauriae” [Crimea, Simeiz], on Rhus coriaria, 1 June 1910, Schirajewsky [Tranzschel & Serebr., Mycoth. Ross. 250] (LE 40410). Isolectotypes: Tranzschel & Serebr., Mycoth. Ross. 250, e.g. BPI 438115, CUP 40252, CUP-F-(M.R. 0250), E 417841, LE 40411, 40412, 158670.
Host range and distribution: On Rhus coriaria, Searsia (glutinosa [Rhus glutinosa], pyroides [Rhus villosa auct., R. vulgaris]), Anacardiaceae, Africa (Ethiopia), Caucasus (Armenia, Georgia), Europe (France, Portugal, Ukraine).
Notes: The name Cercospora marmorata was introduced by Tranzschel in the exsiccata “Tranzschel & Serebr., Mycoth. Ross. 250”, but without any description (nom. nud.). Mycoth. Ross., fasc. V, no. 201–250 was listed and annotated in “Just’s Botanischer Jahresbericht 40 (Abt. 1): 371 (1912)” and “Hedwigia 53: 95 (1913)”, but in both cases without added diagnosis or description of C. marmorata. The first valid publication of this species dates from Saccardo’s (1931) introduction of the “combination” Cercosporina marmorata which was, de facto, a validation since he added a Latin description of this species.
Passalora myracrodruonis (Inácio & Dianese) U. Braun & Crous, Mycosphaerella Anam. 1: 461 (2003).
(Fig. 7)
Fig. 7.
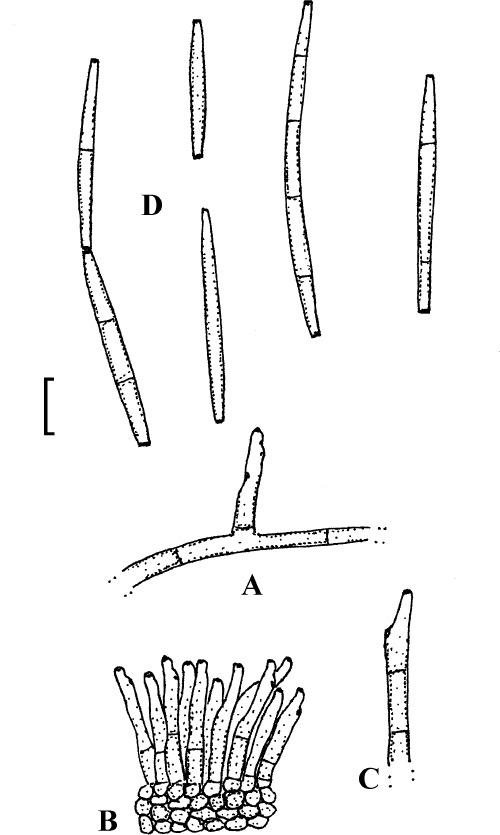
Passalora myracrodruonis (based on Inácio & Dianese 1999: 257, figs 1–3, 258, fig. 5–8).). A. Solitary conidiophore arising from a superficial hypha. B. Conidiophore fascicle. C. Conidiophore. D. Conidia. Bar = 10 μm.
Basionym: Mycovellosiella myracrodruonis Inácio & Dianese, Mycotaxon 72: 253 (1999).
Illustration: Inácio & Dianese (1999: 257, figs 1–3, 258, fig. 5–8).
Description: Leaf spots amphigenous, to 15 mm diam, circular to irregular, confluent, dark brown, later necrotic with a pale greyish centre. Mycelium internal and external; hyphae light brown, septate, 2–4 μm wide, thin-walled, smooth, superficial hyphae mainly hypophyllous, emerging through stomata, arising from stromata, often climbing leaf hairs. Stromata amphigenous, about 15–65(–90) μm diam, subepidermal, erumpent, brown, cells 3–8 μm diam, textura angularis. Conidiophores in dense fascicles, arising from stromata and solitary, arising from superficial hyphae, lateral, erect, straight, subcylindrical to somewhat geniculate-sinuous, simple or occasionally branched, about 20–70 × 2–4 μm, 0–7-septate, brown, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, sympodial, with conspicuous conidiogenous loci, thickened and darkened. Conidia solitary or often in chains, subcylindrical, fusiform to narrowly obclavate, straight to curved, 15–85 × 2–5 μm, 0–4(–6)-septate, light olivaceous to pale brown, thin-walled, smooth, ends obtuse, attenuated to short obconically truncate, hila thickened and darkened-refractive.
Holotype: Brazil: Brasília, DF, Asa Norte, Novacap, Viveiro 2, on Myracrodruon urundeuva, 12 May 1995, C. A. Inácio 292 (UB (col. mycol.) 8767). Paratype (topotype, from 7 June 1995): UB (col. mycol.) 8610.
Host range and distribution: On Myracrodruon urundeuva, Anacardiaceae, South America (Brazil, Brasília, DF).
Passalora pithoragarhensis U. Braun & Crous, Mycosphaerella Anam. 1: 465 (2003).
(Fig. 8)
Fig. 8.
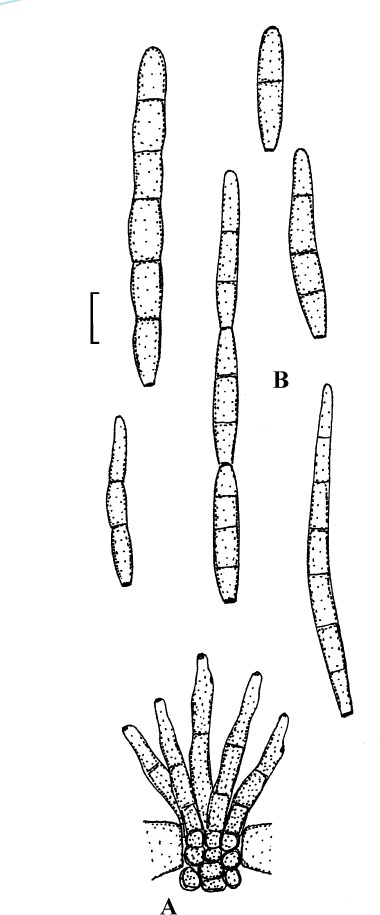
Passalora pithoragarhensis (K(M) IMI 282665, holotype). A. Conidiophore fascicle. B. Conidia. Bar = 10 μm.
Synonym: Phaeoramularia pithoragarhensis Kamal & P. Naraiyan, Indian Phytopathol. 39: 198 (1986); nom. inval. (Art. 40.1).
Literature: Kamal (2010: 133).
Illustration: Kamal & Naraiyan (1986: 199, fig. 1).
Description: Leaf spots amphigenous, subcircular to angular-irregular, to 15 mm diam, brown. Caespituli usually hypophyllous, effuse, velvety, brown. Mycelium internal. Stromata small to well-developed, substomatal, erumpent, medium to dark olivaceous or brown. Conidiophores in small to large fascicles, divergent to dense, arising from stromata, through stomata, erect, subcylindrical to somewhat geniculate-sinuous, 10–75 × 3–8 μm, 0–5-septate, pale brown, thin-walled, smooth; conidiogenous cells integrated, terminal, occasionally conidiophores reduced to conidiogenous cells, conidiogenous loci conspicuous, barely to slightly thickened and darkened. Conidia solitary or in short chains, obclavate-cylindrical, subclavate, short conidia ellipsoid-cylindrical, 15–135 × 4–11.5 μm, 0–10-septate, pale olivaceous, thin-walled, smooth, apex obtuse to attenuated, base obconically truncate, hila barely to slightly thickened and darkened.
Holotype: India: Uttar Pradesh: Pithoragarh, on Searsia parviflora [Rhus parviflora], Anacardiaceae, Nov. 1983, P. Naraiyan (K(M) IMI 282665).
Host range and distribution: Only known from the type collection.
Notes: In the original publication, Kamal & Naraiyan (1986) cited two collections (GPU PH 16 and IMI 282665) but failed to designate holotype material, so that the name Phaeoramularia pithoragarhensis was not validly published according to Art. 40.1. Crous & Braun (2003) examined syntype material from IMI and validated this species name under Passalora.
Passalora rhoina U. Braun & Crous, Mycosphaerella Anam. 1: 352 (2003).
(Fig. 9)
Fig. 9.
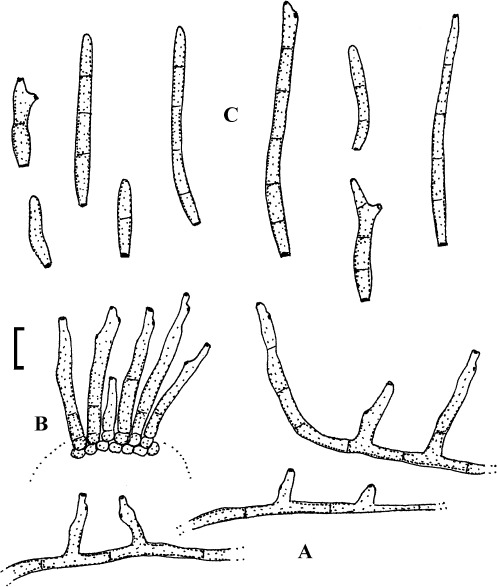
Passalora rhoina (based on Hsieh & Goh 1990: 21, fig. 9). A. Superficial hyphae with solitary conidiophores. B. Conidiophore fascicle. C. Conidia. Bar = 10 μm.
Basionym: Mycovellosiella rhois Goh & W.H. Hsieh, Trans. Mycol. Soc. Republ. China 2: 135 (1987); as “(Sawada & Katsuki) Goh & W.H. Hsieh”, nom. nov. (Art. 58), non Passalora rhois (E. Cast.) U. Braun & Crous, 2003.
Synonyms: Cercospora rhois Sawada & Katsuki, Special Publ. Coll. Agric. Natl. Taiwan Univ. 8: 225 (1958); nom. illeg. (Art. 53.1), non C. rhois E. Cast., 1942 [type: Taiwan: Nantou, Hsinyi, on Rhus chinensis var. roxburghii, 24 Aug. 1944, K. Sawada (NTU-PPE, hb. Sawada)].
?Venturia rhois Sawada, Special Publ. Coll. Agric. Natl. Taiwan 8: 73 (1959), nom. inval (Art. 39.1) [holotype: Taiwan: Nantou, Hsinyi, on Rhus chinensis var. roxburghii, 23 Aug. 1944, K. Sawada (NTU-PPE, hb. Sawada); isotype: K(M) IMI 174953].
?Mycosphaerella rhois C.C. Chen, Bot. Bull. Acad. Sin. 8: 140 (1967); as “(Sawada & Katsuki) C.C. Chen”, nom. nov. (Art. 58).
?Mycosphaerella rhois Sivan., Biblioth. Mycol. 59: 117 (1977); as ‘(Sawada) Sivan.’, nom. nov. (Art. 58), nom. inval. (Art. 39.1) and nom. illeg. (Art. 53.1), non M. rhois C.C. Chen 1967.
Cercospora rhoina Sivan., Bitun. Ascom. Anamorphs: 192 (1984); nom. illeg. (Art. 53.1), non C. rhoina Cooke & Ellis 1878.
Literature: Hsieh & Goh (1990: 20), Sivanesan (1984: 192), Guo et al. (2003: 14–15).
Illustration: Hsieh & Goh (1990: 21, fig. 9), Guo et al. (2003: 14, fig. 3).
Description: Leaf spots amphigenous, forming indefinite pale brown discolorations on the upper leaf surface, below forming distinct greyish brown spots, angular, somewhat floccose, 2–7 mm diam. Caespituli amphigenous, more abundant below. Stromata lacking or developed on the upper side, irregularly shaped, to 65 μm diam, dark brown. Mycelium internal and external; superficial hyphae branched, septate, 2.5–4 μm wide, pale olivaceous to pale brown, thin-walled, smooth. Conidiophores in small to moderately large fascicles, arising from epiphyllous stromata, and solitary, arising from superficial hyphae, lateral and terminal, erect to decumbent, subcylindrical to usually geniculate-sinuous, unbranched or occasionally branched, to 70 μm long and 4–5 μm wide, shorter conidiophores aseptate, longer ones septate, pale olivaceous brown, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, conidiogenous loci conspicuous, somewhat thickened and darkened. Conidia solitary or in chains, cylindrical or subcylindrical, short conidia ellipsoid-fusiform, straight to curved, 20–100 × 4–5 μm, (0–)1–7-septate, occasionally constricted at the septa, subhyaline to pale olivaceous brown, thin-walled, smooth, apex obtuse, base rounded to short obconically truncate, hila slightly thickened and darkened.
Holotype: Taiwan: Nantou, Hsinyi, on Rhus chinensis var. roxburghii, 24 Aug. 1944, K. Sawada (NTU-PPE, hb. Sawada).
Host range and distribution: On Rhus chinensis var. roxburghii [semialata var. roxburghii], Anacardiaceae, Taiwan.
Notes: The genetic connection between Passalora rhoina and associated mycosphaerella-like sexual morphs is unproven, uncertain, and requires molecular and experimental confirmation. Descriptions in the literature differ and are confusing. Sivanesan (1984), based on Mycosphaerella rhois C.C. Chen, described the sexual morph of P. rhoina as follows: Ascomata amphigenous, but mostly epiphyllous, mycosphaerella-like, to 160 μm diam; asci 45–60 × 7–9.5 μm; ascospores 17.5–24 × 3–4 μm, hyaline to light yellow. Aptroot (2006: 19, fig. 762; 175) examined isotype material of Venturia rhois, described much smaller ascospores (11–13 × 2.5–3 μm), and stressed that this species, pertaining to Mycosphaerella sect. Caterva, is morphologically indistinguishable from M. subradians.
Passalora rhois (E. Castell.) U. Braun & Crous, Mycosphaerella and Anam. 1: 353 (2003).
(Fig. 10)
Fig. 10.
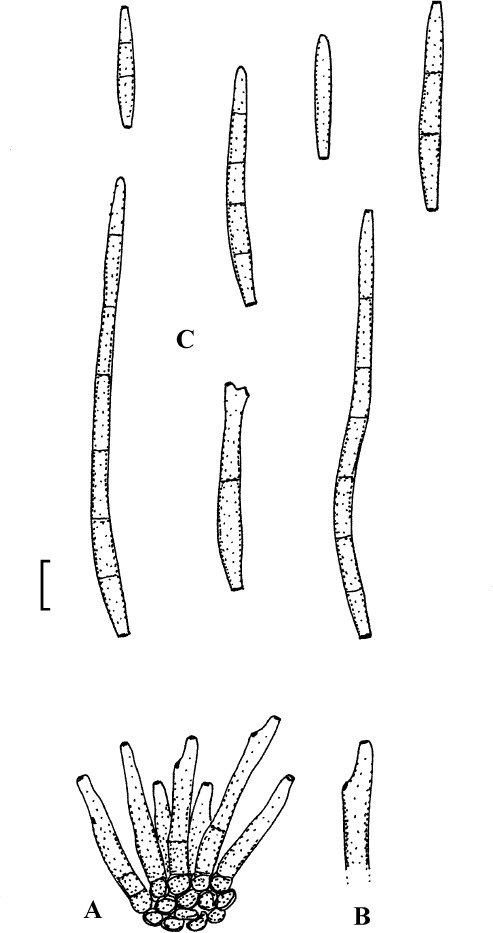
Passalora rhois (K(M) IMI 100210, lectotype). A. Conidiophore fascicle. B. Conidiophore. C. Conidia. Bar = 10 μm.
Basionym: Cercospora rhois E. Castell., Nuovo Giorn. Bot. Ital., N.S., 49: 29 (1942).
Synonym: Phaeoramularia rhois (E. Castell.) Deighton, in Ellis, More Dematiaceous Hyphomycetes: 317 (1976).
Literature: Chupp (1954: 40), Ellis (1976: 316), Deighton (1979: 36), Guo et al. (2003: 131–132).
Illustrations: Ellis (1976: 316, fig. 239B), Deighton (1979: 36, fig. 19), Guo et al. (2003: 132, fig. 81).
Leaf spots amphigenous, 1–4 mm diam, subcircular to irregular, brown to dark brown, centre becoming greyish. Caespituli amphigenous, mostly hypophyllous, punctiform, scattered, dark brown. Mycelium internal. Stromata developed, substomatal to immersed, 10–50 μm diam, brown. Conidiophores in small to moderately large fascicles, arising from stromata, loose to usually dense, emerging through stomata or erumpent, erect, straight, subcylindrical to somewhat geniculate-sinuous, unbranched, 10–40(–90) × 3–6 μm, 0–2-septate, medium olivaceous to brownish, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, conidiogenous loci thickened and darkened, about 1.5–2 μm diam. Conidia catenate, occasionally in branched chains, cylindrical or subcylindrical, straight to curved, 20–120 × 3–5 μm, 0–7-septate, pale to medium olivaceous, olivaceous brown or brownish, thin-walled, smooth, ends rounded to somewhat attenuated, hila 1.5–2 μm wide.
Lectotype (designated here, MycoBank, MBT204887): Ethiopia: Addis Ababa, Achachi, along river Piccolo, on Searsia pyroides [Rhus vulgaris, villosa auct.], 3 Jan. 1938, E. Castellani (K(M) IMI 100210). Isolectotype: BPI 440800.
Host range and distribution: On Rhus chinensis var. roxburghii, Searsia (glutinosa subsp. abyssinica [Rhus glutinosa subsp. abyssinica, Rhus petitiana], pyroides), Anacardiaceae, Africa (Ethiopia), Asia (China).
Passalora rhois-aromaticae U. Braun, sp. nov.
MycoBank MB816981
(Fig. 11)
Fig. 11.
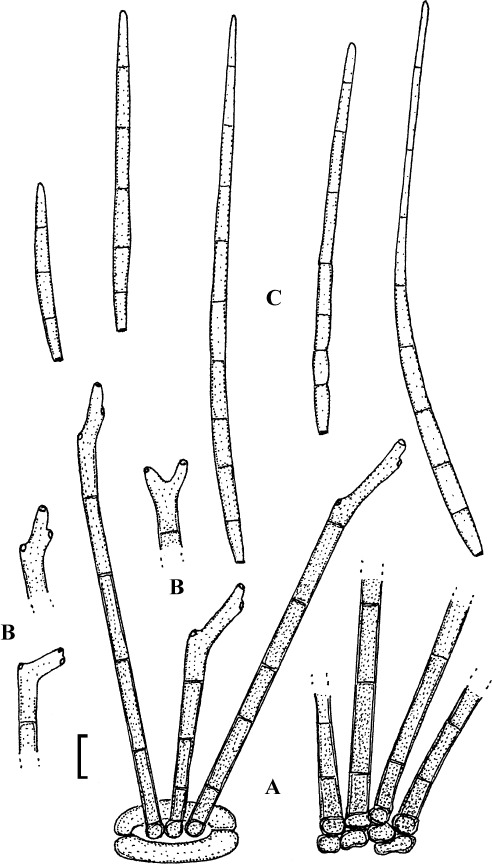
Passalora rhois-aromaticae (BPI 440685, holotype). A. Conidiophore fascicles. B. Conidiophore tips. C. Conidia. Bar = 10 μm.
Etymology: Named after the host species, Rhus aromatica.
Diagnosis: Differs from Passalora pithoragarhensis in having much narrower consistently solitary conidia, 20–180 × 3–5 μm, (0–)2–18-septate.
Description: Leaf spots amphigenous, angular-irregular, 1–10 mm diam, dark brown, margin indefinite. Caespituli hypophyllous, inconspicuous. Mycelium internal. Stromata absent or only with small aggregations of a few swollen hyphal cells, 10–25 μm diam, brown. Conidiophores in divergent fascicles, 2–10, arising from internal hyphae or substomatal aggregations of swollen hyphal cells, emerging through stomata, erect, straight subcylindrical to geniculate-sinuous in the upper fertile portion, unbranched, (15–)30–100 × 3–6 μm, (0–)1–7-septate, pale to medium olivaceous or olivaceous brown throughout or paler towards the tip, wall thin to slightly thickened, smooth; conidiogenous cells integrated, terminal, 10–40 μm long, conidiogenous loci conspicuous, slightly thickened, darkened-refractive. Conidia solitary, obclavate-cylindrical, straight to curved, 20–180 × 3–5 μm, (1–)2–18-septate, distance between septa 5–15 μm, occasionally somewhat constricted at some septa, subhyaline to pale olivaceous or olivaceous brown, smooth or almost so, apex obtuse to subacute, base short to long obconically truncate, 1.5–2.5 μm wide, hila slightly thickened, darkened-refractive.
Holotype: USA: Wisconsin, Dane County, Madison, on Rhus aromatica, 11 Jul. 1951, H. C. Greene (BPI 440685).
Host range and distribution: Only known from the type collection.
Notes: Passalora rhois-aromaticae is morphologically quite distinct from all other described cercosporoid species on hosts of the genus Rhus and allied genera and undoubtedly a new undescribed species. The type material of the new species was originally identified and deposited as “Cercospora rhoina”. However, the true generic affinity of the new species on Rhus aromatica is unclear and can in future only be clarified on the base of molecular analyses. Following the traditional morphological concept of cercosporoid genera, the fungus on Rhus aromatica can currently only be placed in Passalora s. lat. owing to a combination of conspicuous conidiogenous loci and pigmented conidia. Based on consistently internal mycelium, fasciculate conidiophores emerging through stomata, and conidia formed singly, this species belongs to a morphological complex of cercosporoid fungi around Passalora s. str. and Cercosporopidium. Passalora s. lat. (sensu Crous & Braun 2003) is polyphyletic. Morphological traits traditionally used for the discrimination of genera and morphological groups within this complex are not clearly connected with phylogenetic groupings, i.e. morphology is almost meaningless and reliable allocations to genus depend in future on phylogenetic analyses.
Pseudocercospora
Key to species of Pseudocercospora, Scolecostigmina and Stigmina on Anacardiaceae
1 Conidiophores in large sporodochial fascicles, arising from well-developed, large stromata, 15–200 μm diam, proliferation percurrent, with distinct annellations; conidiophores and conidia mostly distinctly verruculose (scolecostigmina/stigmina-like) ............................................................................................................................... 2
Conidiophores sympodially proliferating, rarely sympodial mixed with a few percurrent proliferations, but distinct annellations not formed; conidia mostly smooth or almost so (Pseudocercospora) .............................. 6
2 (1) Conidia ellipsoid-ovoid to oblong, 18–35 × 8–11.5 μm, with 1–4 transverse septa and occasionally a single oblique or longitudinal septum; on Rhus tomentosa ......................................................................... Stigmina pulviniformis
Conidia cylindrical, only 3.5–6 μm wide or scolecosporous, mostly obclavate-cylindrical, length exceeding 40 μm, pluriseptate ...................................................................................................................... 3
3 (2) Conidia cylindrical, (10–)15–45(–55) × 3.5–6 μm, (0–)3(–5)-septate; on Sclerocarya birrea subsp. caffra ............................................................................................................................................... Stigmina knoxdaviesii
Conidia obclavate-cylindrical, longer, exceeding 50 μm, pluriseptate ............................................................................. 4
4 (3) Conidia very long, 85–180 × 5.5–12 μm, strongly curved-sigmoid; on Searsia pyroides ............... Stigmina curvispora
Conidia shorter, 20–130 μm, straight or only slightly curved .......................................................................................... 5
5 (4) Conidiophores short, 5–25 × 3–5 μm, 0–1(–2)-septate; conidia 20–80 × 3–6 μm; on Mangifera indica .................................................................................................... Scolecostigmina mangiferae
Conidiophores longer and broader, 30–50 × 5–8 μm, 1–2-septate; conidia longer and broader, 45–130 × 6–8 μm; on Rhus discolor ................................................................................................. Stigmina rhois
6 (1) Mycelium in vivo only internal; superficial hyphae with solitary conidiophores absent (occasionally with a few hypophyllous superficial hyphae, but without conidiophores); conidiophores strictly fasciculate or sporodochial .................................................................................................... 7
Mycelium in vivo internal and external; superficial hyphae with solitary conidiophores developed .............................. 14
7 (6) Stromata large, 20–125 μm diam; conidiophores numerous, dense, forming sporodochia; on Cotinus and Rhus s. lat. spp. .................................................................................................................. P. rhoina
Stromata smaller, to about 50 μm diam; on other hosts .................................................................................................. 8
8 (7) Conidiophores 10–60 μm long, 0–3(–4)-septate, often branched, distinctly geniculate-sinuous, often with swellings and constrictions; on Lithraea spp. ................................................................... P. phaeochlora
Conidiophores shorter, 5–35 μm, 0–1-septate, unbranched, not strongly geniculate-sinous, usually without distinct constrictions and swellings; on other hosts ......................................................................... 9
9 (8) Conidia rather short, 10–50 μm, only 1–4-septate ........................................................................................................ 10
Conidia longer, at least partly exceeding 50 μm, and at least partly with more than 4 septa ........................................ 11
10 (9) Conidia 1.5–3.5 μm wide, subhyaline or only very pale olivaceous; on Anacardium excelsum .................. P. rhinocarpi
Conidia broader, 3.5–5(–6) μm; on Spondias mombin ................................................... P. mombin var. venezuelensis
11 (9) Conidia hyaline; on Cotinus coggygria ................................................................................................................. P. cotini
Conidia subhyaline, pale olivaceous to olivaceous brown; on other hosts .................................................................... 12
12 (11) Stromata lacking or small; conidiophores fasciculate; conidia olivaceous brown; on Nothopegia dalzelli ........................................................................................................................................................... P. nothopegiae
Stromata well-developed, 15–55 μm diam; conidiophores in small sporodochial conidiomata; conidia subhyaline to pale olivaceous or pale olivaceous brown; on other hosts ................................................... 13
13 (12) Caespituli epiphyllous; on Comocladia spp. ............................................................................................. P. comocladiae
Caespituli amphigenous, but mainly hypophyllous; on Spondias spp. ...................................... P. mombin var. mombin
14 (6) Stromata lacking or small, 10–25 μm diam ................................................................................................................... 15
Stromata well-developed, 10–90 μm diam .................................................................................................................... 18
15 (14) Leaf spots indistinct; conidiophores 10–40 μm long; conidia ± cylindrical, 25–60 × 3.5–4.5 μm, (1–)2–4(–5)-septate; on Schinus spp. .......................................................................................................... P. schini
Leaf spots distinct; conidiophores longer, exceeding 50 μm; conidia obclavate-cylindrical, longer, narrower or wider, 1–11-septate; on other hosts .................................................................................................... 16
16 (15) Conidia 30–70 × 3–9 μm; on Rhus and Toxicodendron ................................................................................ P. infuscans
Conidia narrower, 2.5–4.5 μm; on other hosts .............................................................................................................. 17
17 (16) Conidia 40–125 × 3–4.5 μm; on Anacardium spp., Central and South America ............................................. P. anacardii
Conidia narrower, 40–80 × 2.5–3.5 μm; on Mangifera indica, Asia, India .............................................. P. baruipurensis
18 (14) Stromata large, 50–90 μm diam; conidia 40–175 × 4–7.5 μm, 3–17(–25)-septate; on Rhus and Toxicodendron ........................................................................................................................................................................ P. rhois
Stromata smaller, about 10–50 μm diam; conidia narrower, 1.5–5.5 μm wide ............................................................. 19
19 (18) Conidia usually narrowly subcylindrical, 20–65 × 1.5–3 μm, hila 1–2 μm wide; on Pistacia spp. .................. P. pistaciae
Conidia obclavate-cylindrical, broader, 2.5–6.5 μm; on other hosts ............................................................................. 20
20 (19) Conidia (2.5–)3–5.5(–6.5) μm, hila 1.5–3 μm wide; on Rhus and Toxicodendron spp. .................................. P. rhoicola
Conidia narrower, 2.5–4 μm, hila 1.5–2 μm wide; on other hosts ................................................................................. 21
21 (20) Leaf spots reddish brown, finally leaving shot-holes; conidia to 105 μm long, with to 12 septa; on Lannaea coromandelia .......................................................................................................................... P. odinae
Leaf spots medium to dark brown, later paler with darker margin, without shot-hole symptoms; conidia to 70 μm long, with to 7 septa; on Cotinus coggygria ........................................................................ P. cotini
Tabular key to Pseudocercospora, Scolecostigmina and Stigmina species on Anacardiaceae according to host genera
Anacardium
1 Mycelium internal and external; superficial hyphae with solitary conidiophores developed; fasciculate conidiophores 40–140 × 3–6 μm; conidia obclavate, 40–125 × 3–4.5 μm, 3–11-septate, pale olivaceous to olivaceous brown; on Anacardium spp. .............................................. P. anacardii
Mycelium internal, superficial hyphae with solitary conidiophores not formed; conidiophores uniformly short and narrow, 5–20 × 1.5–2.5 μm; conidia obclavate-cylindrical, shorter and narrower, 10–50 × 1.5–3.5 μm, 1–4-septate, subhyaline to pale olivaceous; on Anacardium excelsum ............................................................................................................................................................... P. rhinocarpi
Comocladia
A single species ................................................................................................................................................... P. comocladiae
Cotinus
A single species ............................................................................................................................................................... P. cotini
Lithraea
A single species .................................................................................................................................................... P. phaeochlora
Lannea
A single species ............................................................................................................................................................. P. odinae
Mangifera
1 Conidiophores in sporodochial conidiomata with well-developed stromata; conidiophores short, 5–25 μm, 0–1(–2)-septate, subcylindrical to conical or ampulliform, percurrently proliferating, with conspicuous annellations; conidia 3–6 μm wide, at least partly verruculose ................................................................................................................................... Scolecostigmina mangiferae
Conidiophores fasciculate; stromata lacking; conidiophores much longer, 25–130 μm, pluriseptate, proliferation sympodial, annellations lacking; conidia narrower, 2.5–3.5 μm ........................................................................................................................................................ P. baruipurensis
Nothopegia
A single species ................................................................................................................................................... P. nothopegiae
Rhus s. lat. (including Searsia and Toxicodendron)
1 Conidiophores in large sporodochial fascicles, arising from well-developed, large stromata, 15–200 μm diam, proliferation percurrent, with distinct annellations; conidiophores and conidia mostly distinctly verruculose (scolecostigmina/stigmina-like) ...................................................................... 2
Conidiophores sympodially proliferating, rarely sympodial mixed with few percurrent proliferations, distinct annellations not formed; conidia mostly smooth or almost so (Pseudocercospora s. str.) ........................... 4
2 (1) Conidia ellipsoid-ovoid to oblong, 18–35 × 8–11.5 μm, with 1–4 transverse septa and occasionally a single oblique or longitudinal septum; on Rhus tomentosa ............................................... Stigmina pulviniformis
Conidia scolecosporous, mostly obclavate-cylindrical, length exceeding 40 μm, pluriseptate ....................................... 3
3 (2) Conidiophores 4–6 μm wide; conidia very long, 85–180 × 5.5–12 μm, strongly curved-sigmoid; on Searsia pyroides ................................................................................................................. Stigmina curvispora
Conidiophores 5–8 μm wide; conidia somewhat shorter and narrower, 45–130 × 6–8 μm, straight to slightly curved; on Rhus discolor ...................................................................................... Stigmina rhois
4 (1) Stromata lacking or very small; conidia 30–70 × 3–9 μm ............................................................................. P. infuscans
Stromata well-developed, 10–125 μm diam .................................................................................................................... 5
5 (4) Mycelium internal, superficial hyphae with solitary conidiophores not developed (occasionally with a few hypophyllous superficial hyphae, but without conidiophores); stromata large, 20–125 μm diam; conidiophores in large sporodochial conidiomata; conidia 20–90(–120) × (2–)2.5–5(–5.5) μm ................................................................................................ P. rhoina
Mycelium internal and external; superficial hyphae with solitary conidiophores developed; stromata either smaller, 10–50 μm diam, or, if large, conidia 4–7.5 μm wide .......................................................... 6
6 (5) Stromata large, 50–90 μm diam; conidia 40–175 × 4–7.5 μm, 3–17(–25)-septate ............................................... P. rhois
Stromata smaller, about 10–50 μm diam; conidia narrower, (2.5–)3–5.5(–6.5) μm wide, 2–12-septate .......... P. rhoicola
Pistacia
A single species .......................................................................................................................................................... P. pistaciae
Schinus
A single species ............................................................................................................................................................... P. schini
Sclerocarya
A single species ....................................................................................................................................... Stigmina knoxdaviesii
Searsia, see Rhus
Spondias
1 Conidia 20–65(–85) × (1–)1-5–3.5 μm, 2–7-septate ..................................................................P. mombin var. mombin
Conidia shorter and broader, 12–40 × 3.5–5 μm, with few septa, 1–3(–4)-septate ........ P. mombin var. venezuelensis
Toxicodendron, see Rhus
Pseudocercospora species on Anacardiaceae
Pseudocercospora anacardii E. Castell. & Crasulli, Rivista Agric. Subtrop. Trop. 75: 103 (1981).
(Fig. 12)
Fig. 12.
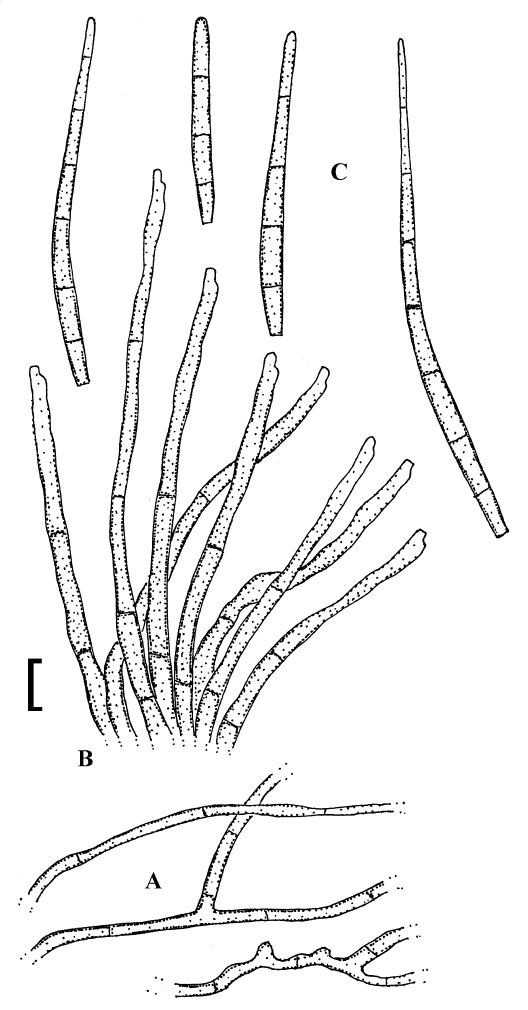
Pseudocercospora anacardii (CUP-MG-000218, holotype). A. Superficial hyphae. B. Conidiophore fascicle. C. Conidia. Bar = 10 μm.
Synonym: Cercospora anacardii A.S. Mull. & Chupp, Arq. Inst. Biol. Veg. 1: 214 (1935); nom. inval. (Art. 39.1) [holotype: Brazil: Minas Gerais: Ponte Nova, on Anacardium occidentale, 15 Aug. 1930, A. S. Muller 218 (CUP-MG-000218)].
Literature: Chupp (1954: 38), Teixeira (1988), Crous & Braun (2003: 56), O’Farrell et al. (2002).
Illustration: Castellani & Casulli (1981: Pl. 1)
Description: Leaf spots at first indistinct, later angular-irregular on the upper leaf surface, 1–4 mm diam, reddish brown, on the lower leaf surface visible by effuse fructification. Caespituli hypophyllous, effuse, at first grey, greyish olivaceous, later brown to dark brown. Mycelium internal and external; superficial hyphae emerging through stomata, sparingly branched, straight to sinuous, occasionally torulose, 1–3 μm wide, hyaline, subhyaline to pale olivaceous, septate, thin-walled, smooth. Stromata lacking or small, 10–25 μm diam, olivaceous brown. Conidiophores in small to moderately large fascicles, loose, arising from internal hyphae or stromata, through stomata, occasionally solitary, arising from superficial hyphae, erect, straight, subcylindrical to usually distinctly sinuous or geniculate-sinuous, unbranched or only rarely branched, 40–140 × 3–6 μm, pluriseptate throughout, uniformly olivaceous brown to medium brown or tips somewhat paler, thin-walled, smooth to faintly rough; conidiogenous cells integrated, terminal, 10–35 μm long, conidiogenous loci inconspicuous or subdenticulate, but wall always unthickened and not darkened. Conidia solitary, obclavate, straight to curved, 40–125 × 3–4.5 μm, 3–11-septate, pale olivaceous to olivaceous brown, thin-walled, smooth to faintly rough, apex obtuse to subacute, base short obconically truncate, 1.5–2 μm wide, hila neither thickened nor darkened.
Holotype: Brazil: Minas Gerais: Ponte Nova, on Anacardium occidentale, 15 Aug. 1930, A. S. Muller 218 (CUP-MG-000218).
Host range and distribution: On Anacardium (humile, occidentale), Anacardiaceae, Africa (Guinea-Bissau, Tanzania, Zambia), Australia, Central and South America (Brazil, Panama).
Pseudocercospora baruipurensis K.K. Sarbajna, Indian Phytopathol. 43: 23 (1990).
(Fig. 13)
Fig. 13.
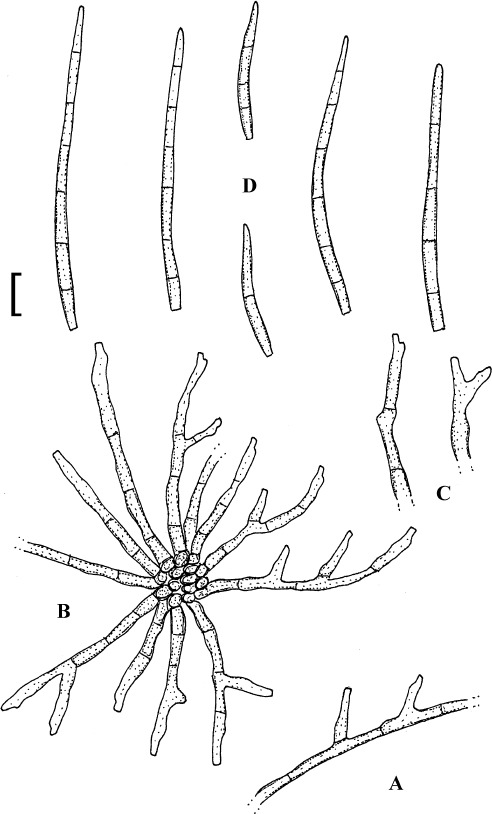
Pseudocercospora baruipurensis (K(M) 303912, isotype). A. Superficial hypha with solitary conidiophores. B. Conidiophore fascicle. C. Conidiophores. D. Conidia. Bar = 10 μm.
Literature: Kamal (2010: 153).
Illustration: Sarbajna (1990: 24, fig. 2).
Description: Leaf spots amphigenous, distinct, angular-irregular, vein-limited, 2–8 mm diam, often confluent, dark to reddish brown. Caespituli hypophyllous, evenly scattered, light to dark brown. Mycelium internal and external; internal hyphae 2–3 μm wide, superficial hyphae hypophyllous, emerging through stomata, arising from the base of conidiophore fascicles, branched, septate, 2–4 μm wide, pale olivaceous, smooth, thin-walled. Stromata absent or almost so. Conidiophores in loose fascicles, 3–15, arising from internal hyphae or small hyphal aggregations, through stomata, erect to decumbent, straight, subcylindrical to geniculate-sinuous, simple or frequently branched, 25–130 × 3–6.5 μm, 4–10-septate, pale brown, thin-walled, smooth or conidiophores solitary, arising from superficial hyphae, lateral, sometimes terminal, shorter than fasciculate conidiophores and with fewer septa or aseptate; conidiogenous cells integrated, terminal or conidiophores sometimes reduced to conidiogenous cells, 10–25 μm long, conidiogenous loci neither thickened nor darkened. Conidia solitary, obclavate-cylindrical, straight to slightly curved, 45–80 × 2.5–3.5 μm, 1–10-septate, pale olivaceous, thin-walled, smooth, apex subobtuse to subacute, base obconically truncate, 1–2 μm wide, hila neither thickened nor darkened.
Holotype: India: West Bengal: Baruipur, on Mangifera indica, 12 Mar. 1886, K. K. Sarbajna (PPC 3811 = Presidency College Calcutta, Botany Department). Isotype: K(M) 303912.
Host range and distribution: Only known from the type collection.
Notes: In the introduction of the original publication, Sarbajna (1990) stated ‘Holotype specimens are deposited in the herbarium, Botany Department, Presidency College, Calcutta (PPC)’. This is sufficient to conform with the provisions of Art 40.6, although the word “type or holotype” is not cited under the listed type collections of P. baruipurensis.
Pseudocercospora comocladiae (Petr. & Cif.) Deighton, Mycol. Pap. 140: 142 (1976).
(Fig. 14)
Fig. 14.
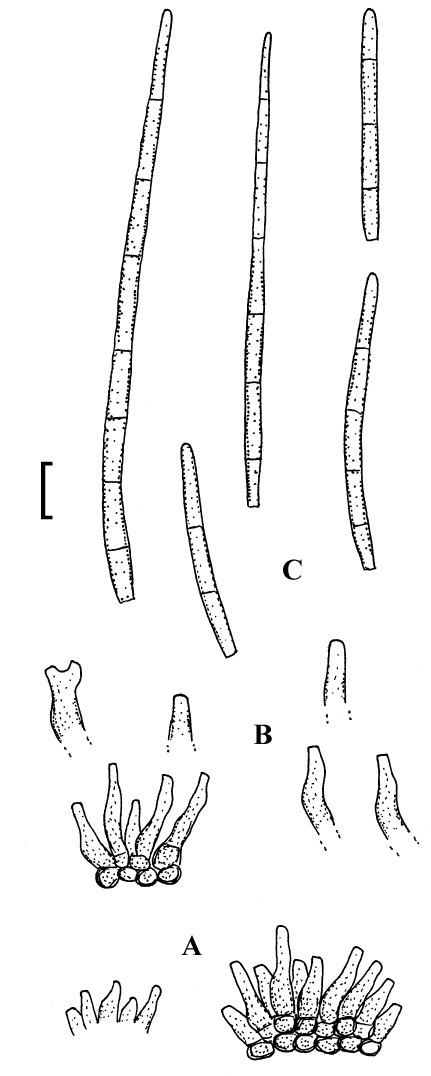
Pseudocercospora comocladiae (W Krypt 1973-0011010, lectotype). A. Conidiophore fascicles. B. Conidiophores. C. Conidia. Bar = 10 μm.
Basionym: Cercospora comocladiae Petr. & Cif., Ann. Mycol. 30: 308 (1932).
Literature: Chupp (1954: 39), Castañeda Ruiz & Braun (1989: 51).
Illustration: Castañeda Ruiz & Braun (1989: 49, pl. 5, fig. 29).
Description: Leaf spots amphigenous, subcircular to angular-irregular, 1–5 mm diam or confluent and larger, brown with narrow darker margin on the lower leaf surface, brown, sometimes reddish brown to almost black on the upper side, later becoming pale, greyish white, with dark, border, occasionally somewhat raised. Caespituli epiphyllous, finely punctiform, scattered, brown. Mycelium internal. Stromata immersed, 15–50 μm diam, dark brown. Conidiophores in small to moderately large fascicles, arising from stromata, mostly dense, erect, straight to strongly geniculate-sinuous, unbranched, subyclindrical to somewhat conical, 5–15 × 3–4 μm, aseptate, pale to medium olivaceous brown, thin-walled, smooth; conidiophores reduced to conidiogenous cells, conidiogenous loci inconspicuous, unthickened, not darkened. Conidia solitary, cylindrical, obclavate-cylindrical, subacicular, straight to somewhat curved, 25–80 × 2–3.5 μm, 2–10-septate, subhyaline to olivaceous, thin-walled, smooth, apex obtuse to subacute, base short obconically truncate or rounded, 1.5–2 μm wide, hila unthickened, not darkened.
Lectotype (designated here, MycoBank, MBT204888): Dominican Republic: Province Santiago: Santiago, Coesta de Piedras, 200 m alt., on Comocladia dodonaea, 9 Dec. 1930, E. L. Ekman 3880 (W Krypt 1973-0011010). Isolectotypes: NY 936983, S-F21822.
Host range and distribution: On Comocladia (dentata, dodonaea), Anacardiaceae, West Indies (Cuba, Dominican Republ.).
Notes: The material deposited at NY is marked as type, but the date is given as “1. Dec. 1930”. It is not quite clear if this material represents an isolectotype or topotype material.
Pseudocercospora cotini (Katsuki & Tak. Kobay.) Deighton, Trans. Brit. Mycol. Soc. 88: 389 (1987).
(Fig. 15)
Fig. 15.
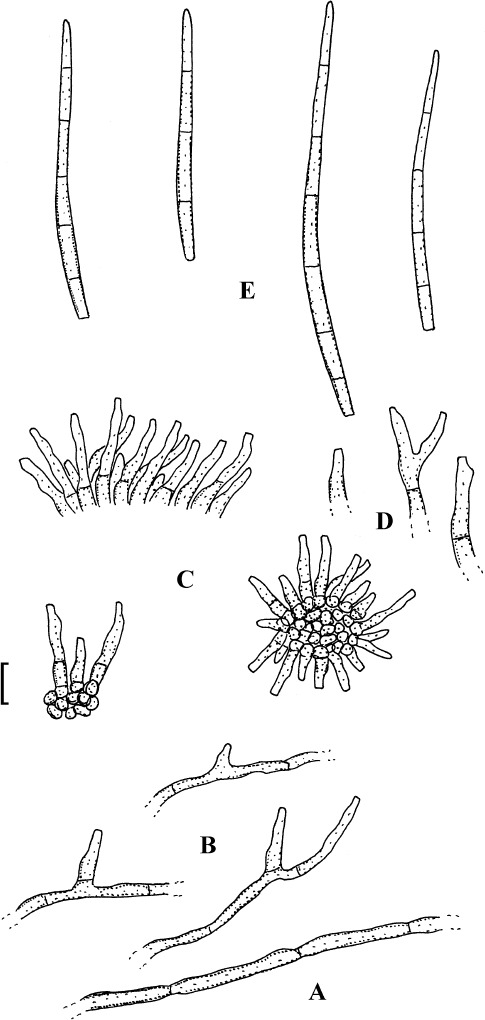
Pseudocercospora cotini (BPI 440738). A. Superficial hypha. B. Superficial hyphae with solitary conidiophores. C. Conidiophore fascicles. D. Conidiophores. E. Conidia. Bar = 10 μm.
Basionym: Cercospora cotini Katsuki & Tak. Kobay., Trans. Mycol. Soc. Japan 17: 274 (1976).
Literature: Crous & Braun (2003: 141).
Illustration: Katsuki & Kobayashi (1976: 275, fig. 3)
Description: Lesions caulicolous and foliicolous, amphigenous, at first minute, 0.5–2 mm diam, later forming indefinite to angular-irregular spots, 3–10 mm diam, often vein-limted, on the upper side sometimes somewhat raised, medium to dark brown, later paler, with deep brown margin, along midribs and on stems elliptical to oblong, 1–5 × 0.5–1 mm, finally confluent. Caespituli amphigenous, mainly epiphyllous, punctiform, effuse, dark brown. Mycelium internal or partly external; superficial hyphae confined to the lower leaf surface, sparingly branched, septate, 1–4 μm wide, subhyaline to pale olivaceous or olivaceous brown, thin-walled, smooth. Stromata epiphyllous, immersed, intra- to subepidermal, subglobose to somewhat irregularly shaped, 10–50 μm diam, olivaceous brown, cells globose, sublobose to somewhat irregular, hypophyllously absent or smaller, substomatal to immersed. Conidiophores on the upper leaf surface in moderately large fascicles, arising from stromata, erumpent, below in smaller fascicles, arising from internal hyphae or smaller stromata, through stomata or erumpent, below also solitary, arising from superficial hyphae, erect, straight, subcylindrical or attenuated towards the tip, somewhat geniculate-sinuous, unbranched, 5–35 × 1.5–4 μm, aseptate or sparingly septate, subhyaline to pale olivaceous or olivaceous brown, usually paler towards the tip, apex often subhyaline, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores often reduced to conidiogenous cells, proliferating sympodially, conidiogenous loci inconspicuous or visible as truncate tip or shoulder, but always unthickened and not darkened, 1.5–2 μm. Conidia solitary, cylindrical to obclavate-cylindrical, straight to curved, 25–70 × 2.5–4 μm, 2–7-septate, hyaline, subhyaline to pale olivaceous or very pale olivaceous brown, thin-walled, smooth, tips subacute to subobtuse, base short to long obconically truncate, 1–2 μm wide, hila unthickened and not darkened.
Holotype: Japan: Fukuoka Prefecture: Forest Experimental Station, on Cotinus coggygria, 20 Sept. 1974, S. Ogawa (TFM:FPH-4184); ex-holotype culture MAFF 410088.
Host range and distribution: On Cotinus coggygria, Anacardiaceae, Asia (Japan), North America (USA, Alabama, Florida, Texas).
Notes: North American specimens on Cotinus coggygria, previously referred to as Cercospora rhoina, belong to Pseudocercospora cotini. A collection from Alabama (Tuskegee, Sep. 1900, G. W. Carver, BPI 440738) has been examined and revised. Records of “C. rhoina” from Texas and “Cercospora sp.” from Florida (Alfieri et al. 1984) seem to belong to P. cotini as well. Another collection from Alabama on Cotinus coggygria proved to be a new species of Passalora s. lat.
Pseudocercospora infuscans (Ellis & Everh.) U. Braun, Monograph of Cercosporella, Ramularia and allied genera (phytopathogenic hyphomycetes) 2: 402 (1998).
(Fig. 16)
Fig. 16.

Pseudocercospora infuscans (NY 838246, lectotype). A. Conidiophore fascicle. B. Superficial hyphae with solitary conidiophores. C. Conidiophore tips. D. Conidia. Bar = 10 μm.
Basionym: Cercospora infuscans Ellis & Everh., Proc. Acad. Nat. Sci. Philadelphia 43: 90 (1891).
Misapplied name: Cercospora toxicodendri sensu Chupp (1954: 43) p.p.
Literature: Saccardo (1892: 639), Chupp (1954: 43, p.p.), Braun (1998: 402).
Illustrations: Braun (1998: 403, fig. 656).
Description: Leaf spots amphigenous, angular-irregular, 1–6 mm diam, dull greenish or different shades of brown, margin indefinite. Caespituli amphigenous, usually hypophyllous, effuse, brown. Mycelium internal and external; superficial hyphae sparingly branched, septate, 2–4 μm wide, subhyaline to pale olivaceous, thin-walled, smooth. Stromata absent or almost so. Conidiophores in small, loose fascicles, 2–4, emerging through stomata, and solitary, arising from superficial hyphae, lateral, occasionally terminal, erect to decumbent, subcylindrical to clavate, barely to slightly geniculate-sinuous, simple, rarely branched, 5–100 × 3–8 μm, 0–6-septate, pale olivaceous, olivaceous brown to medium brown, wall thin to somewhat thickened, smooth; conidiogenous cells integrated, terminal, sometimes conidiophores reduced to conidiogenous cells, 5–25 μm long, proliferation sympodial, occasionally percurrent, conidiogenous loci unthickened, not darkened, often truncate, sometimes subdenticulate. Conidia solitary, obclavate-cylindrical, broadly fusiform, straight to curved, 30–70 × 3–9 μm, 3–7(–10)-septate, pale olivaceous to brownish, thin-walled, smooth, apex obtuse to subacute, base obconically truncate, 1.5–3.5 μm wide, hila unthickened, not darkened.
Lectotype (designated here, MycoBank, MBT204889): USA: Delaware: Porter’s Station, on Rhus venenata, 9 Oct. 1890, A. Commons 1621 (NY 838246). Isolectotype: NY 838247.
Host range and distribution: On Rhus (glabra, venenata, vernix, Rhus sp.), Toxicodendron (pubescens, radicans), Anacardiaceae, North America (USA, Delaware, Florida, Indiana, Iowa, Kansas, Maryland, Massachusetts, New Jersey, North Carolina, Texas, Wisconsin).
Notes: Cercospora infuscans is a true species of Pseudocercospora. Chupp (1954), who confused various cercosporoid fungi on Rhus, reduced this name to synonymy with C. toxicodendri. The complicated taxonomy and nomenclature of this species has been discussed in detail by Braun (1998).
Pseudocercospora mombin (Petr. & Cif.) Deighton, Mycol. Pap. 140: 148 (1976).
var. mombin
(Fig. 17a)
Fig. 17.
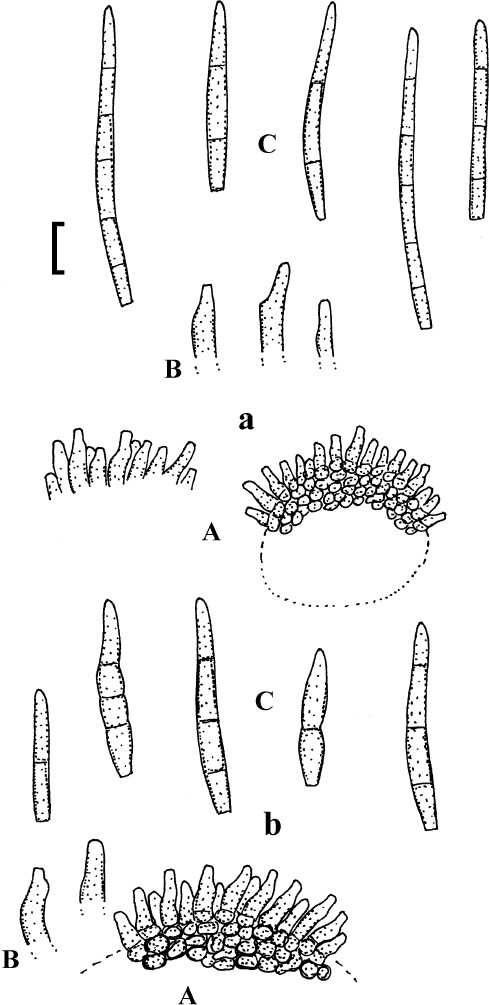
17a. Pseudocercospora mombin var. mombin (W Krypt 1973-0010017, lectotype). A. Conidiophore fascicles. B. Conidiophores. C. Conidia. 17b. P. mombin var. venezuelensis (HAL 2157 F, holotype). A. Conidiophore fascicle. B. Conidiophores. C. Conidia. Bar = 10 μm.
Basionym: Cercospora mombin Petr. & Cif., Ann. Mycol. 30: 322 (1932).
Literature: Chupp (1954: 41), Braun et al. (1999: 102), Crous & Braun (2003: 279), Kamal (2010: 199), Phengsintham et al. (2013: 116–117).
Illustration: Phengsintham et al. (2013: 117, figs 74–75).
Description: Leaf spots amphigenous, subcircular to angular-irregular, 1–40 mm diam, pale to dark brown, greyish brown, finally dull grey to greyish white, margin indefinite or with narrow dark brown, sometimes somewhat raised border. Caespituli amphigenous, mainly hypophyllous, scattered to gregarious, punctiform, dark brown to almost blackish. Mycelium internal; hyphae intercellular, 2–4 μm wide, sparingly branched, septate, subhyaline, smooth. Stromata 15–55 μm diam, substomatal to immersed, subglobose to planate, brown to dark brown, cells 2–6 μm diam. Conidiophores in small to moderately large fascicles (2–18), arising from stromata, through stomata or erumpent, loose to mostly dense, erect, straight to somewhat curved, unbranched, rarely branched at the base, subcylindrical to somewhat conical or ampulliform, 5–25(–30) × 1.5–5 μm, 0–1-septate, subhyaline to pale olivaceous brown throughout or tips paler, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, 5–25 μm long, conidiogenous loci inconspicuous or visible as truncate tip, but always unthickened and not darkened. Conidia solitary, cylindrical to obclavate-cylindrical, straight to curved, 20–65(–85) × (1–)1.5–3.5 μm, 2–7-septate, subhyaline to pale olivaceous or olivaceous brown, thin-walled, smooth, apex subobtuse to subacute, base truncate to short obconically truncate, 0.5–2 μm wide, hila unthickened, not darkened.
Lectotype (designated here, MycoBank, MBT204890): Dominican Republic: Province Santiago: Valle de Ciboa, Hato del Yaque, on Spondias mombin, 1 May 1931, R. Ciferri. 4018 (W Krypt 1973-0010017). Isolectotype: PAV. Topotypes (1 Jan 1931): BPI 438565A–B.
Host range and distribution: On Spondias (dulcis [cytherea], mombin, purpurea), Anacardiaceae, Central and South America (Brazil, Panama, Venezuela), Asia (Philippines, Thailand), North America (Mexico), Oceania (Samoa), West Indies (Cuba, Dominican Republic).
Notes: The record from Mexico (on S. purpurea) refers to a collection deposited as BPI 438566, and the record from Samoa is included in Dingley et al. (1981).
var. venezuelensis U. Braun & Urtiaga, Feddes Repert. 119: 494 (2008).
(Fig. 17b)
Illustration: Braun & Urtiaga (2008: 495, fig. 7).
Description: Differs from P. mombin var. mombin in having broader, 1–3(–4)-septate conidia, 12–40 × 3–5(–6) μm.
Holotype: Venezuela: Lara State: Barquisimeto, on Spondias mombin, Anacardiaceae, Jan. 2007, R. Urtiaga (HAL 2157 F).
Host range and distribution: Only known from the type collection.
Pseudocercospora nothopegiae (Ramakr. et al.) Deighton, Mycol. Pap. 140: 149 (1976).
(Fig. 18)
Fig. 18.
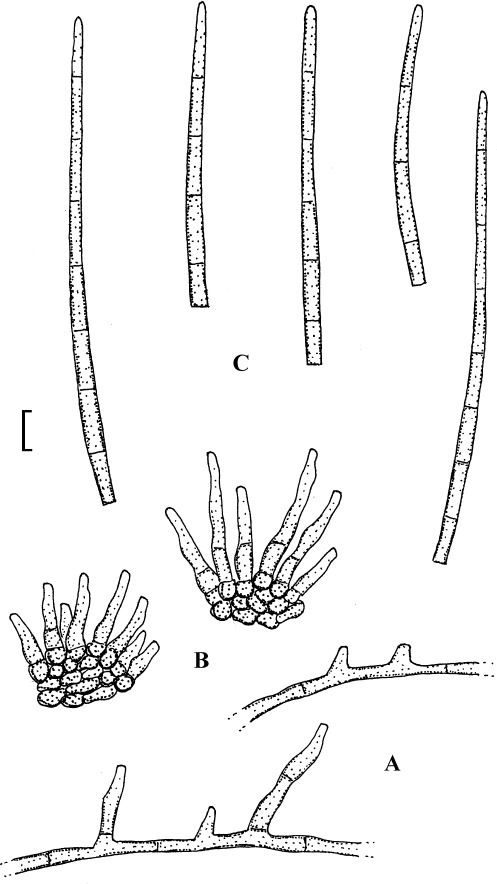
Pseudocercospora nothopegiae (K(M) IMI 62837, lectotype). A. Superficial hyphae with solitary conidiophores. B. Conidiophore fascicles. C. Conidia. Bar = 10 μm.
Basionym: Cercospora nothopegiae Ramakr. et al., Proc. Indian Acad. Sci., Sect. B, 37: 94 (1953).
Literature: Vasudeva (1963: 152), Crous & Braun (2003: 292), Kamal (2010: 203).
Exsiccatae: Herb. Crypt. Ind. Orient Exs. III. Indian Cercosporae, Fasc. 1, no. 31.
Description: Leaf spots amphigenous, subcircular to angular-irregular, 0.5–5 mm diam, gregarious, at first olivaceous brown, later darker, dingy blackish brown, finally paler, greyish brown to dingy grey, margin indefinite or narrow and darker. Caespituli hypophyllous, inconspicuous to punctiform, dark brown. Mycelium internal and external; superficial hyphae sparingly branched, 1.5–4 μm wide, septate, subhyaline, pale olivaceous to olivaceous brown, thin, walled, smooth. Stromata immersed to somewhat erumpent, small to medium in size, 10–40 μm diam, sometimes confluent and larger, olivaceous brown, cells 2–6 μm diam. Conidiophores in small to moderately large fascicles, loose to moderately dense, arising from stromata, or solitary arising from superficial hyphae, lateral, erect, straight to somewhat flexuous and geniculate-sinuous, subcylindrical or attenuated towards the apex, unbranched, 5–35 × 2–4.5 μm, 0–2-septate, pale olivaceous to olivaceous brown, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, 5–25 μm long, conidiogenous loci mostly truncate, but neither thickened nor darkened. Conidia solitary, subcylindrical, obclavate-cylindrical, subacicular, straight to curved, (15–)30–100 × 2–4.5 μm, (1–)3–9-septate, subhyaline, pale olivaceous to medium olivaceous brown, thin-walled, smooth, apex obtuse to subacute, base truncate to somewhat obconically truncate, (1.5–)2–2.5(–3) μm wide, hila unthickened, not darkened.
Lectotype (designated here, MycoBank, MBT204891): India: Tamil Nadu: Chennai (Madras), Naduvattam, on Nothopegia dalzellii, Anacardiaceae, 24 Feb. 1952, T. S. Ramakrishnan & K. V. Srinivasan [Herb.Crypt. Ind. Orient Exs. III. Indian Cercosporae, Fasc. 1, no. 31] (K(M) IMI 62837). Isolectotypes: Herb. Crypt. Ind. Orient Exs. III. Indian Cercosporae, Fasc. 1, no. 31 (e.g. HCIO).
Host range and distribution: Only known from the type collection.
Pseudocercospora odinae K.K. Sarbajna, Indian Phytopathol. 43: 21 (1990).
(Fig. 19)
Fig. 19.
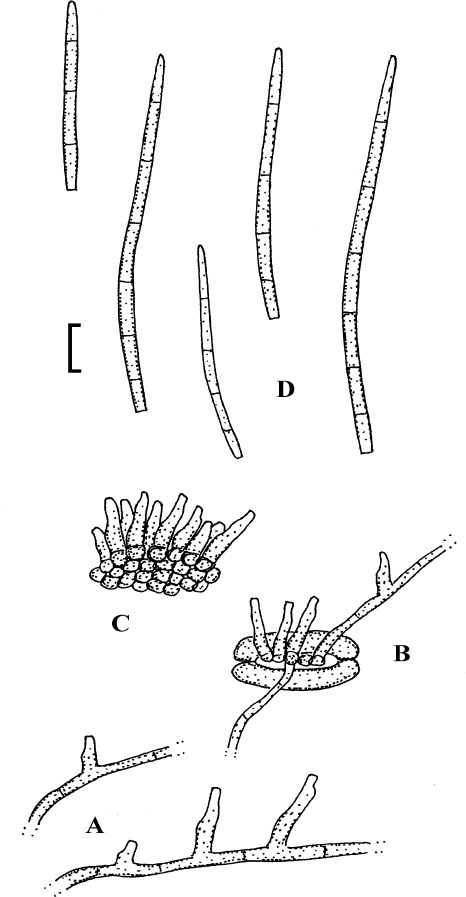
Pseudocercospora odinae (K(M) IMI 309962, isotype). A. Superficial hyphae with solitary conidiophores. B. Conidophores and hyphae emerging through a stoma. C. Conidiophore fascicle. D. Conidia. Bar = 10 μm.
Illustration: Sarbajna (1990: 21, fig. 1).
Description: Leaf spots amphigenous, circular or subcircular, vein-limited, 3–8 mm diam, reddish brown, finally leaving shot-holes. Caespituli hypophyllous, evenly scattered, light brown. Mycelium internal and external; internal hyphae branched, septate, 2–2.5 μm wide, hyaline; superficial hyphae arising from the base of conidiophore fascicles, branched, septate, pale olivaceous, thin-walled, smooth. Stromata developed, substomatal, globose or subglobose, compact, dark brown, composed of swollen, brownish hyphal cells. Conidiophores in small to large fascicles, 5–45, arising from stromata, divergent or conidiophores solitary, arising from superficial hyphae, erect, straight to curved, usually unbranched, 6.5–30.5 × 2.5–3.5 μm, 0–3-septate, pale olivaceous, wall somewhat thickened, smooth; conidiogenous cells integrated, terminal or conidiophores sometimes reduced to conidiogenous cells, subdenticulate, but conidiogenous loci unthickened, not darkened. Conidia solitary, obclavate-cylindrical, straight to curved, 30–105 × 3.5–4 μm, 3–12-septate, pale olivaceous, thin-walled, smooth, apex subotuse or subacute, base obconically truncate, about 1.5–2 μm wide, hila unthickened, not darkened
Holotype: India: West Bengal: 24 Parganas (South), Amtala, on Lannea coromandelica [Odina woodier], 11 Aug. 1986, K. K. Sarbajna (PCC 3251 = Presidency College Calcutta, Botany Department). Isotype: K(M) IMI 309962.
Host range and distribution: Only known from the type collection.
Note: See nomenclatural comment under Pseudocercospora baruipurensis.
Pseudocercospora phaeochlora (Speg.) U. Braun et al., Fungal Diversity 6: 31 (2001).
(Fig. 20)
Fig. 20.
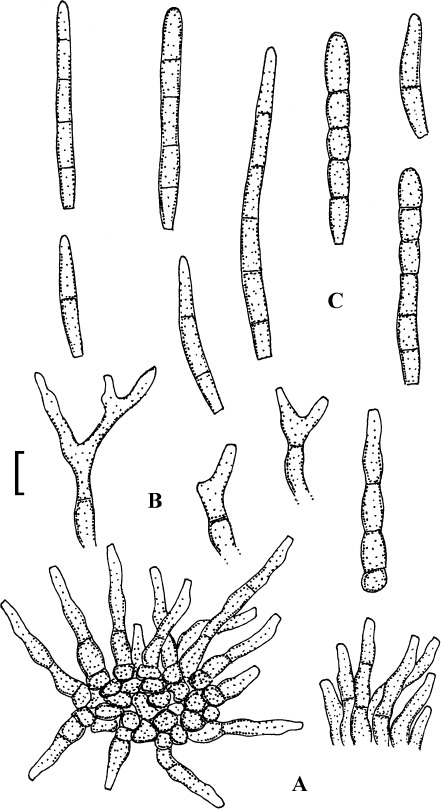
Pseudocercospora phaeochlora (LPS 947, holotype). A. Conidiophore fascicles. B. Conidiophores. C. Conidia. Bar = 10 μm.
Basionym: Cercospora? phaeochlora Speg., Anales Mus. Nac. Hist. Nat. Buenos Aires 20: 441 (1910).
Literature: Saccardo (1913: 1414), Chupp (1954: 41), Braun (2000: 68), Crous & Braun (2003: 319).
Illustration: Braun et al. (2001: 29, fig. 10).
Description: Leaf spots absent to angular-irregular, 1–7 mm diam, or oblong, to 10 mm, sometimes confluent and larger, on the upper leaf surface visible as pale yellowish ochraceous discolorations, on the lower leaf surface reddish to dark brown, finally sooty due to abundant fungal colonies, margin indefinite, sometimes vein-limited. Caespituli hypophyllous, effuse, loose to dense, olivaceous to sooty. Mycelium internal. Stromata small to fairly large, occasionally confluent, substomatal to intraepidermal, immersed to erumpent, about 10–50 μm diam, olivaceous brown. Conidiophores in small to moderately large fascicles, divergent to dense, arising from stromata, through stomata or erumpent, erect, flexuous, geniculate-sinuous, often branched, 10–60 × 2–5 μm, width irregular, often with constrictions and swellings, continuous to 1–3(–4)-septate, pale yellowish brown to olivaceous or medium brown, smoth, thin-walled; conidiogenous cells integrated, terminal or conidiophores occasionally reduced to conidiogenous cells, 10–25 μm long, conidiogenous loci inconspicuous. Conidia solitary, cylindrical to obclavate, straight to curved, (15–)30–80(–90) × (2–)3–5(–5.5) μm (according to Chupp l.c., to 150 μm long), 1–7-septate, sometimes constricted at the septa, pale to medium olivaceous brown or yellowish brown, thin-walled, smooth, apex obtuse, often broadly rounded, base rounded, truncate to obconically truncate, 2–2.5 μm wide, hila unthickened, not darkened.
Holotype: Argentina: Buenos Aires, Jardín Botanico, on Lithraea brasiliensis, 28 Apr. 1906, C. Spegazzini (LPS 947).
Host range and distribution: On Lithraea (brasiliensis, molleoides, venenosa [caustica]), Anacardiaceae, South America (Argentina, Chile).
Pseudocercospora pistaciae (Chupp) Crous & U. Braun, Mycotaxon 78: 338 (2001).
(Fig. 21)
Fig. 21.
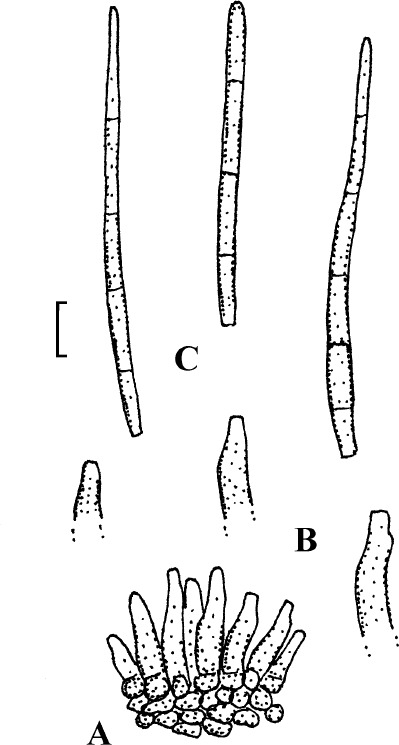
Pseudocercospora pistaciae (CUP 40569, lectotype). A. Conidiophore fascicle. B. Conidiophores. C. Conidia. Bar = 10 μm.
Basionym: Cercospora pistaciae Chupp, Monograph of Cercospora: 41 (1954).
Synonym: Cercospora pistaciae Sawada ("pistaceae"), Special Publ. Coll. Agric. Natl. Taiwan Univ. 8: 224 (1959), nom. nud. (Art. 38.1).
Literature: Chupp (1954: 41), Hsieh & Goh (1990: 20), Crous & Braun (2003: 326).
Illustrations: Crous & Braun (2001: 335, fig. 6).
Description: Leaf spots at first inconspicuous, later amphigenous, irregularly shaped, 0.5–3 mm diam, sometimes confluent and larger, brown to greyish brown, margin distinct, dark brown. Caespituli amphigenous, punctiform, brown. Mycelium internal and external; superficial hyphae branched, 3–4 μm wide, light brown, thin-walled, smooth. Stromata 10–60 μm diam, substomatal to immersed, brown. Conidiophores in small to moderately large fascicles, usually dense, arising from stromata, through stomata or erumpent, or solitary, arising from superficial hyphae, lateral, erect, straight to somewhat curved, subcylindrical-conical, unbranched, 5–15 × 2–3.5 μm, aseptate, subhyaline, pale olivaceous to light brown, thin-walled, smooth; conidiophores reduced to conidiogenous cells, conidiogenous loci inconspicuous. Conidia solitary, narrowly subcylindrical to somewhat obclavate-cylindrical, straight to curved, 20–65 × 1.5–3 μm, 3–5-septate, subhyaline to pale olivaceous, thin-walled, smooth, apex subobtuse to subacute, base obconically truncate, 1–2 μm wide, hila unthickened, not darkened.
Lectotype (designated here, MycoBank, MBT204892): USA: Texas: Bexar County, San Antonio, on Pistacia vera, Sep. 1931, G. T. Ratliffe (CUP 40569). Isolectotype: BPI 439746.
Host range and distribution: On Pistacia (atlantica [mutica], ?chinensis, vera), Anacardiaceae, ?Asia (China, Taiwan), North America (USA, Maryland, Texas).
Notes: Hsieh & Goh (1990) examined original material of C. pistaciae Sawada deposited at NTU-PPE (Taiwan: Hsinchu, 4 Dec. 1928, K. Sawada), but did not find any sporophores on the leaves. Collections on Pistacia atlantica from Texas and material found in Maryland, USA, are deposited at BPI.
Pseudocercospora rhinocarpi U. Braun & Crous, in Crous & Braun, Mycosphaerella Anam. 1: 351 (2003).
(Fig. 22)
Fig. 22.
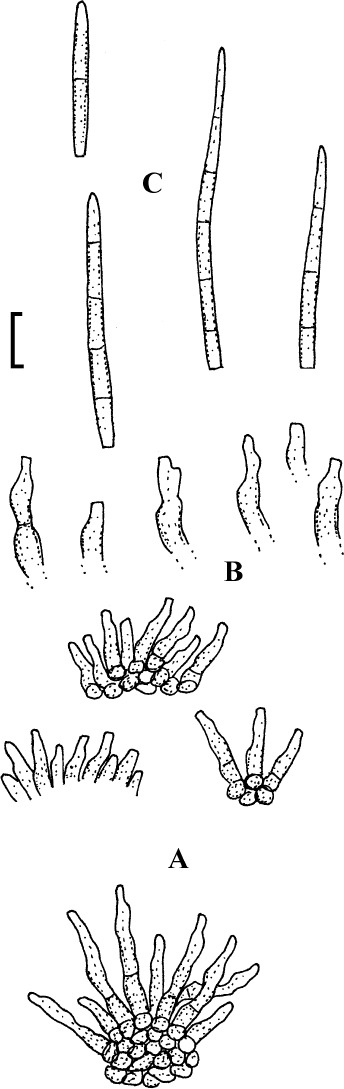
Pseudocercospora rhinocarpi (CUP-VZ 2047, holotype). A. Conidiophore fascicles. B. Conidiophores. C. Conidia. Bar = 10 μm.
Synonym: Cercospora rhinocarpi Chupp & A.S. Mull., Bol. Soc. Venez. Ci. Nat. 8: 54 (1942); nom. inval. (Art. 39.1).
Literature: Chupp (1954: 42), Braun & Urtiaga (2013: 201).
Illustration: Crous & Braun (2003: 352, fig. 21).
Leaf spots amphigenous, irregular, 2–8 mm diam, brown to greyish brown, margin indistinct or with a narrow, somewhat raised border. Caespituli amphigenous, delicately punctiform, scattered to aggregated, dark brown. Mycelium immersed. Stromata substomatal or intraepidermal, 10–30 μm diam, yellowish to olivaceous brown, substomatal to intraepidermal. Conidiophores loosely to densely fasciculate, arising from stromata, through stomata or erumpent, erect, flexuous, subcylindrical to geniculate-sinuous, unbranched, 5–20 × 1.5–2.5 μm, 0–1-septate, subhyaline to very pale olivaceous, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, 5–15 μm long, conidiogenous loci inconspicuous. Conidia solitary, narrowly obclavate-subcylindrical, subacicular, 10–50 × 1.5–3.5 μm, 1–4-septate, subhyaline to pale olivaceous, smooth, apex subacute to subobtuse, base truncate to obconically truncate, 1–1.5 μm wide, hila unthickened, not darkened.
Holotype: Venezuela: Aragua: Maracay, on Anacardium excelsum, 26 Nov. 1937, A. S. Muller (CUP-VZ 2047).
Host range and distribution: On Anacardium excelsum [rhinocarpus], Anacardiaceae, South America (Venezuela).
Notes: This is a typical Pseudocercospora with inconspicuous conidiogenous loci. Cercospora rhinocarpi is an invalid name published without Latin description. An English description was published by Chupp (1954), but the validation of this name dates from Crous & Braun (2003).
Pseudocercospora rhoicola U. Braun & C. Nakash., sp. nov.
MycoBank MB816982
(Fig. 23)
Fig. 23.
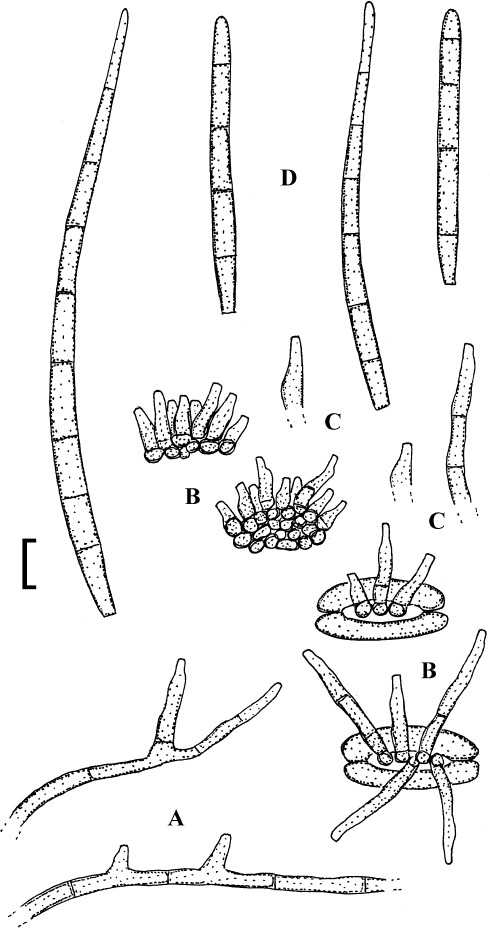
Pseudocercospora rhoicola (CUP 40763, holotype). A. Solitary conidiophores arising from superficial hyphae. B. Conidiophore fascicles. C. Conidiophores. D. Conidia. Bar = 10 μm.
Misapplied names: Cercospora toxicodendri sensu Katsuki (1965: 10), Pseudocercospora toxicodendri sensu Guo & Hsieh (1995: 14) and Guo et al. (1998: 25–26), P. rhoina sensu Sarbajna & Chattopadhyay (1991: 47), P. infuscans p.p. in Crous & Braun (2003: 226).
Etymology: Named after the host genus, Rhus (dweller of Rhus).
Diagnosis: Morphologically comparable to the North American Pseudocercospora infuscans, but stromata developed, 10–50 μm diam, above all on the upper leaf surface, conidia longer and narrower, 30–120 × (2.5–)3–5.5(–6.5) μm.
Holotype: Japan: Tokyo, Murayama reservoir, on Rhus sp., 12 Oct. 1952, E. Kurosawa (CUP 40763).
Literature: Sarbajna & Chattopadhyay (1991: 47, as “P. rhoina”), Guo & Hsieh (1995: 14, as “P. toxicodendri”), Braun (1998: 402), Guo et al. (1998: 25–26, “P. toxicodendri”), Kamal (2010: 215, as “P. rhoina”).
Illustrations: Sarbajna & Chattopadhyay (1991: 48, fig. 3, as “P. rhoina”), Guo & Hsieh (1995: 15, fig. 14, as “P. toxicodendri”), Braun (1998: 403, fig. 656), Guo et al. (1998: 26, fig. 14, as “P. toxicodendri”).
Description: Leaf spots amphigenous, subcircular to usually angular-irregular, sometimes vein-limited, 1–10 mm diam, reddish brown, dark brown to blackish brown, later sometimes greyish brown to greyish white, above all on the upper surface, often paler and less distinct below, margin indefinite. Caespituli amphigenous, subeffuse below, punctiform above, brown to blackish brown. Mycelium internal and external; superficial hyphae on the lower leaf surface, emerging through stomata, arising from stromata or the base of conidiophore fascicles, sparingly branched, septate, 1.5–3(–5) μm wide, subhyaline to pale olivaceous, thin-walled, smooth. Stromata absent, above all on the lower leaf surface, to developed, above all on the upper leaf surface, 10–50 μm diam, medium to dark olivaceous brown, composed of swollen hyphal cells, circular to somewhat irregular in outline, 2–6 μm diam. Conidiophores on the lower leaf surface in small, loose fascicles, about 2–5, emerging through stomata, and solitary, arising from superficial hyphae, lateral, occasionally terminal, on the upper leaf surface in small to moderately large fascicles, arising from stromata, through stomata or erumpent, erect to decumbent, straight, subcylindrical or somewhat narrowed towards the tip, barely to distinctly geniculate-sinuous, simple, rarely branched, 5–65 × 3–6(–7) μm, 0–3(–7)-septate, pale to medium olivaceous or olivaceous brown, wall thin, smooth; conidiogenous cells integrated, terminal, sometimes conidiophores reduced to conidiogenous cells, 5–25 μm long, proliferation sympodial, conidiogenous loci unthickened, not darkened, often truncate, sometimes subdenticulate. Conidia solitary, obclavate-cylindrical, straight to curved, 20–120 × (2.5–)3–5.5(–6.5) μm, 2–12-septate, pale brown or pale olivaceous to olivaceous brown, thin-walled, smooth, apex obtuse to subacute, base obconically truncate, 1.5–3 μm wide, hila unthickened, not darkened.
Host range and distribution: On Rhus (aromatica, chinensis, javanica, Rhus sp.), Toxicodendron orientale [Rhus ambigua], Anacardiaceae, Asia (China; India, West Bengal; Japan).
Notes: Pseudocercospora species on Rhus were notoriously confused and misinterpreted. Above all, the name Cercospora toxicodendri has been used for different cercosporoid fungi (Braun 1998). The North American P. infuscans is morphologically comparable to and possibly confusable with the present new Asian species, but differs in having shorter and wider conidia, 30–70 × 3–9 μm, and lacking stromata. The conidiophores are often subclavate and may proliferate percurrently. Additional Japanese material has been examined: Yamagata, Kamabuchi, on R. javanica, 15 Oct. 1950, T. Kobayashi (TFM:FPH-528).
Pseudocercospora rhoina (Cooke & Ellis) Deighton, Mycol. Pap. 140: 152 (1976); as “rhuina”.
(Fig. 24)
Fig. 24.
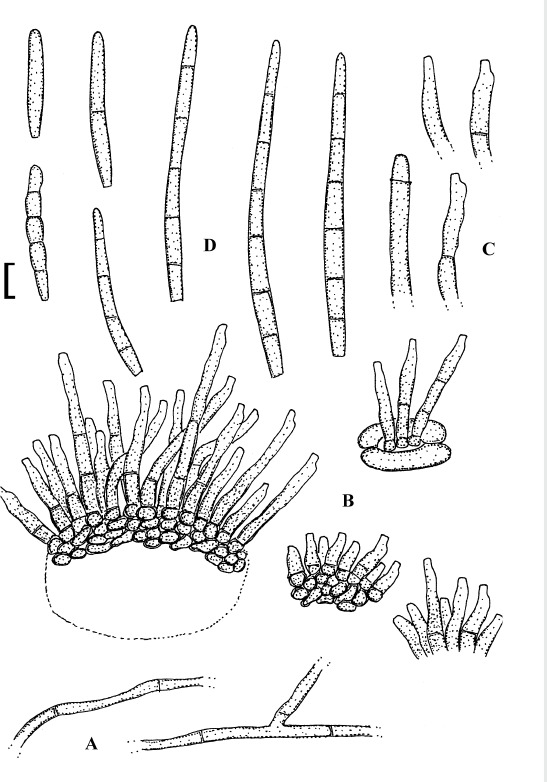
Pseudocercospora rhoina (K(M) 202815, lectotype). A. Superficial hyphae. B. Conidiophore fascicles. C. Conidiophores. D. Conidia. Bar = 10 μm.
Basionym: Cercospora rhoina Cooke & Ellis, Grevillea 6: 89 (1878).
Synonym: Cercospora copallina Cooke, Grevillea 12: 31 (1883) [holotype: USA: South Carolina: Aiken, on Rhus copallina, H. W. Ravenel 586 (K(M) 202807)].
Cercospora rhoina var. nigromaculans Peck, Rep. (Annual) New York State Mus. Nat. Hist. 42: 129 (1889) [holotype: USA: New York: Manor, Long Island, on Rhus copallina, Aug., C. H. Peck (NYS)].
Literature: Saccardo (1886: 467–468), Chupp (1954: 42), Katsuki (1965: 10, as C. toxicodendri), Sarbajna & Chattopadhyay (1991: 47), Guo & Hsieh (1995: 13), Guo et al. (1998: 24), Shin & Kim (2001: 218), Crous & Braun (2003: 353).
Illustrations: Sarbajna & Chattopadhyay (1991: 48, fig. 3), Guo & Hsieh (1995: 14, fig. 13), Guo et al. (1998: 25, fig. 13), Shin & Kim (2001: 219, fig. 100).
Exsiccatae: Barthol., Fungi Columb. 2613, 2809, 4613. Ellis, Fungi Columb. 794. Ellis & Everh., N. Amer. Fungi 1252, 1505. Rabenh., Fungi Eur. Exs. 3682. Ravenel, Fungi Amer. Exs. 586. Seym. & Earle, Econ. Fungi 119. Sydow, Fungi Exot. Exs, 447.
Description: Leaf spots amphigenous, subcircular to angular-irregular, 1–8 mm diam, sometimes vein-limited, brown, reddish brown or even blackish, later centre greyish brown to greyish white, above all on the upper leaf surface, margin at first yellowish, later brown to reddish brown, sometimes with yellowish halo or slightly zonate, finally sometimes thin, dropping out, causing shot-hole symptoms. Caespituli amphigenous, punctiform to pustulate on the upper leaf surface, dark brown to blackish, on the lower leaf surface not pustulate, more delicate, punctiform, scattered. Mycelium internal, occasionally with some superficial hyphae, mainly on the lower leaf surface, emerging through stomata, sparingly branched, septate, thin-walled, smooth, pale olivaceae to brownish, 1.5–4 μm wide. Stromata small to well-developed, large, substomatal to immersed, erumpent, 20–125 μm diam, subglobose to somewhat irregularly shaped or oblong, lacking or smaller on the lower leaf surface, 10–55 μm diam, larger on the upper leaf surface, usually > 50 μm diam, brown to blackish brown, cells 2–7 μm diam, circular to somewhat angular-irregular in outline, wall slightly thickened. Conidiophores on the upper side in well-developed, mostly large and dense fascicles, 10–40, arising from stromata, on the lower side in smaller fascicles, through stomata or erumpent, erect, straight to somewhat curved, subcylindrical or somewhat attenuated towards the tip, to geniculate-sinuous, unbranched, 5–70(–85) × (1.5–)2–6 μm, 0–3-septate, uniformly pale to medium olivaceous to medium olivaceous brown or brown, thin-walled, smooth, occasionally somewhat rough-walled with age; conidiogenous cells, integrated, terminal or conidiophores reduced to conidiogenous cells, mostly 5–40 μm long, proliferation sympodial, rarely percurrent, conidiogenous loci inconspicuous or visible as truncate tip, neither thickened nor darkened. Conidia solitary, obclavate-cylindrical, straight to curved, 20–120(–140) × (2–)2.5–5.5(–6) μm, 2–10(–12)-septate, subhyaline to pale olivaceous or olivaceous brown, thin-walled, smooth, apex obtuse to subacute, base subtruncate to short obconically truncate, 1.5–2.5(–3) μm wide, hila unthickened, not darkened.
Lectotype (designated here, MycoBank, MBT204893): USA: New Jersey: Newfield, on Rhus glabra, 7 June 1877, J. B. Ellis 2656 (K(M) 202815). Isolectotypes: K(M) 202817–202819.
Host range and distribution: On Rhus (aromatica, canadensis, chinensis, copallinum, coriaria, glabra, lanceolata, michauxii [pumila], potaninii, taitensis, trilobata, typhina, vernix, Rhus sp.), Searsia natalensis [Rhus natalensis], Toxicoxdendron [orientale [Rhus ambigua], sempervirens, vernicifluum], Anacardiaceae, Africa (Kenya, Malawi), Asia (China, ?India, Japan, Korea, Taiwan), Europe (Ukraine), North America (Canada; USA, Alabama, Arkansas, California, Connecticut, Delaware, Florida, Illinois, Indiana, Iowa, Louisiana, Maryland, Massachusetts, Minnesota, Mississippi, Missouri, Montana, Nebraska, New Jersey, New York, North Carolina, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, West Virginia, Wisconsin).
Notes: Pseudocercospora rhoina undoubtedly represents a complex of races or even cryptic species which needs to be split on the base of cultures and results of molecular sequence analyses. This widespread species has a broad host range amongst species of Rhus and allied genera and is morphologically rather variable. Collections on Toxicoxdendron orientale [Rhus ambigua] in Japan are, for instance, characterized by having relatively narrow conidia at the lower limit of the variation. It is doubtful that all collections from Africa and Asia are conspecific with North Americal collections. Sarbajna & Chattopadhyay (1991) described and illustrated P. rhoina from India (West Bengal) on Rhus sp. The collection concerned was misidentified and belongs to P. rhoicola. Kamal (2010) listed Rhus aromatica as additional Indian host of P. rhoina. Source and identity of the latter record are unclear and unproven. North American records of P. rhoina on Cotinus coggygria are based on confusions with P. cotini (see notes under P. cotini).
Pseudocercospora rhois Y.L. Guo & X.J. Liu, Acta Mycol. Sin., Suppl. 1: 345 (1986); as “rhoidis”.
(Fig. 25)
Fig. 25.
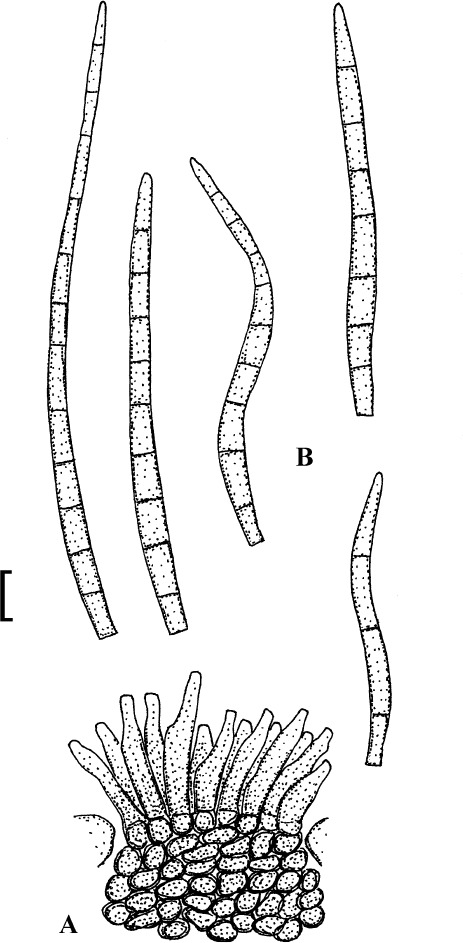
Pseudocercospora rhois (based on Guo & Hsieh 1995: 13, fig. 12). A. Conidiophore fascicle, B. Conidia. Bar = 10 μm.
Literature: Guo & Hsieh (1995: 12), Guo et al. (1998: 23).
Illustrations: Guo (1986: 346, fig. 2), Guo & Hsieh (1995: 13, fig. 12), Guo et al. (1998: 24, fig. 12).
Description: Leaf spots amphigenous, subcircular, 0.5–2 mm diam, often confluent, centre whitish, greyish white to pale brown, margin conspicuous on the upper leaf surface, narrow, dark brown, spots paler below. Caespituli amphigenous, mainly epiphyllous, punctiform, dark. Mycelium internal and partly external; superficial hyphae septate, 2–2.5 μm wide, pale olivaceous. Stromata epiphyllous, immersed, subepidermal, located along the margin of leaf spots, subglobose, 50–90 μm diam, dark brown. Conidiophores in small fascicles emerging through stomata to numerous, in dense fascicles arising from stromata or solitary, arising from superficial hyphae, lateral, erect, straight, subcylindrical, attenuated towards the tip, not or only very slightly geniculate-sinuous, unbranched, 9–37 × 3–5 μm, aseptate, pale brown to brown, thin-walled, smooth; conidiophores reduced to conidiogenous cells, conidiogenous loci inconspicuous. Conidia solitary, obclavate-cylindrical, straight, curved to somewhat sigmoid, 40–175 × 4–7.5 μm, 3–17(–25)-septate, pale to medium brown, thin-walled, smooth, apex obtuse to subacute, base obconically truncate, hila unthickened, not darkened.
Holotype: China: Hubei Province: Shennongjia, Xiaoping, on Toxicodendron sylvestre, 10 Aug. 1984, Y. L. Guo 312 (HMAS 47834).
Host range and distribution: On Rhus hypoleuca, Toxicodendron sylvestre [Rhus sylvestris], Anacardiaceae, Asia (China, Hubei, Sichuan).
Pseudocercospora schini (Syd. & P. Syd.) U. Braun & Crous, in Crous & Braun, Mycosphaerella Anam. 1: 368 (2003).
(Fig. 26)
Fig. 26.
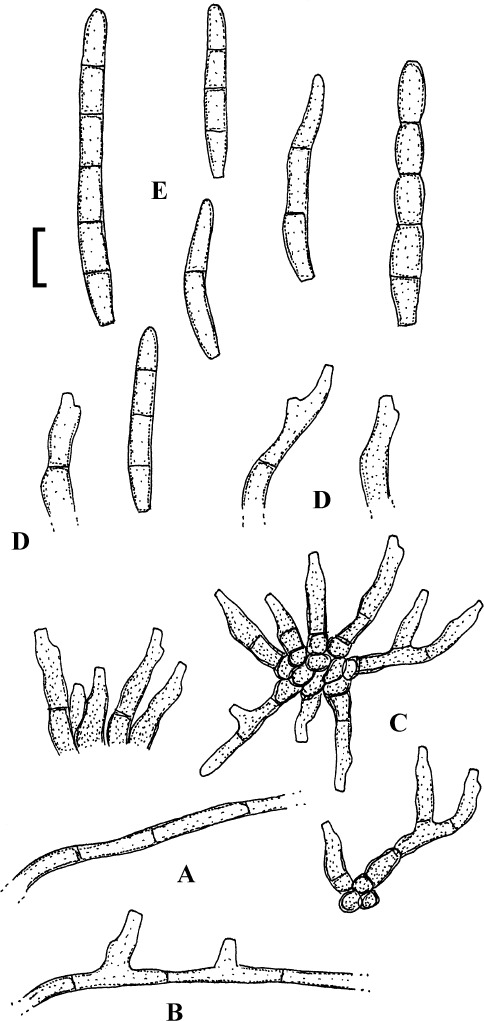
Pseudocercospora schini (CUP 41175, isotype). A. Superficial hypha. B. Superficial hypha with solitary conidiophores. C. Conidiophore fascicles. D. Conidiophores. E. Conidia. Bar = 10 μm.
Basionym: Cercospora schini Syd. & P. Syd., Mem. Herb. Boissier 8(4): 2 (1900).
Literature: Saccardo (1902: 1068), Chupp (1954: 43), Alfieri et al. (1984).
Description: Leaf spots indistinct, forming yellowish discolorations on the upper leaf surface. Caespituli hypophyllous, finely punctiform to effuse, olivaceous. Mycelium internal or partly external, with a few superficial hyphae, septate, sparingly branched, 2–5 μm wide, pale. Stromata lacking or only formed as small aggregations of a few swollen hyphal cells, olivaceous, substomatal. Conidiophores in small to moderately large fascicles, arising from internal hyphae or swollen hyphal cells, through stomata, or conidiophores solitary, arising from superficial hyphae, lateral, occasionally terminal, erect to decumbent, straight, subcylindrical or attenuated towards the tip, moderately geniculate-sinuous, unbranched or occasionally branched, 10–40 × 3–7 μm, 0–3-septate, olivaceous, olivaceous brown or yellowish brown, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, 10–25 μm long, conidiogenous loci inconspicuous, occasionally visible as truncate tip or denticle-like lateral shoulder caused by sympodial proliferation, but always unthickened and not darkened. Conidia solitary, cylindrical or subcylindrical, straight to curved, 25–60 × 3.5–4.5 μm, (1–)2–4(–5)-septate, occasionally somewhat constricted at the septa, yellowish to olivaceous brown, thin-walled, smooth, apex obtuse, base short obconically truncate, about 2 μm wide, hila unthickened, not darkened.
Holotype: Argentina: Province Córdoba: Córdoba, on Schinus polygama, Apr. 1899, T. Stuckert 6805 (S-F20495). Isotype: CUP 41175.
Host range and distribution: On Schinus (polygama [dependens], terebinthifolia), Anacardiaceae, North America (USA, Florida), South America (Argentina, Córdoba).
Doubtful, excluded and insufficiently known species
Phaeoisariopsis pulgaonvensis Dharkar & Subhedar, Geobios 33: 206 (2006).
Illustration: Dharkar & Subhedar (2006: 206, fig. 1).
Description: Infection spots orbicular. Colonies dark brown to black. Mycelium immersed, subhyaline to light brown. Stromata partly immersed, prosenchymatous, brown, to 76.5 μm diam. Conidiophores synnematous along the basal portion and almost mononematous towards the apex, unbranched, septate, olivaceous brown to brown, smooth, thin-walled, geniculate, 60.8–323 μm long and 1.9–3.8 μm thick at the base. Conidia solitary, acropleurogenous, 15.2–41.8 × 11.4–15.2 μm, 1–4-septate, smooth [conidial shape and colour not described, but based on the original illustration obviously broadly ellipsoid-ovoid, pigmented, base and apex rounded].
Holotype: India: Maharashtra: Pulgaon, Wardha District, on Mangifera indica, 25 Aug. 2003, N. S. Dharkar (AMH 8873).
Host range and distribution: Only known from the type collection.
Notes: Based on the morphology and phylogenetic position of its type species, the genus Phaeoisariopsis has been reduced to synonymy with Pseudocercospora (Crous et al. 2006, Braun et al. 2013). However, Phaeoisariopsis pulgaonvensis is a saprobic, non-cercosporoid hyphomycete growing on dead stems of Mangifera indica, at first glance resembling the saprobic, synnematous genus Podosporium, but the genuine generic affinity of P. pulgaonvensis requires a re-examination of type material, which was not available.
Stigmina curvispora Crous & U. Braun, Sydowia 46: 220 (1994).
(Fig. 27)
Fig. 27.
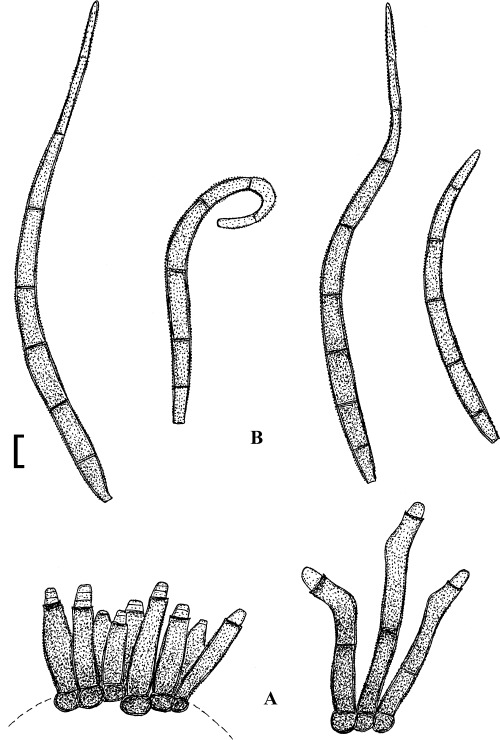
Stigmina curvispora (PREM 51105, holotype). A. Parts of sporodochia with conidiophores. B. Conidia. Bar = 10 μm.
Illustration: Crous & Braun (1994: 221, fig. 10).
Description: Leaf spots amphigenous, scattered, subcircular to irregular, 2–10 mm diam, dark brown. Caespituli epiphyllous, punctiform, dark. Mycelium internal; composed of dark brown, smooth hyphae. Stromata substomatal, 60–80 μm wide and 10–20 μm high, composed of swollen hyphal cells, brown, pseudoparenchymatous. Conidiophores numerous, arising from stromata, forming well-developed, brown sporodochia, 70–90 × 30–50 μm, emerging through stomata, erect, straight to slightly curved or sinuous, unbranched, subcylindrical, doliiform, 10–60 × 4–6 μm, 0–3-septate, brown, thin-walled, verruculose; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, about 10–30 μm long, proliferation percurrent, with conspicuous annellations. Conidia solitary, obclavate, usually strongly curved-sinuous, 85–180 × 5.5–12 μm, 4–9-septate, pale brown, thin-walled, verruculose, apex obtuse to subacute, base obconically truncate, occasionally with minute frill, hila unthickened, not darkened.
Holotype: South Africa: Mpumalanga (Eastern Transvaal): Roodeplaat, Vegetable & Ornamental Plant Research Institute, experimental farm, on Searsia pyroides [Rhus pyroides], Anacadiaceae, Mar. 1988, E. J. van der Linde (PREM 51105).
Host range and distribution: Only known from the type collection.
Notes: This species is scolecostigmina-like. Based on its type species, Scolecostigmina turned out to be a phylogenetically separate genus clustering apart of Pseudocercospora (Crous et al. 2013), but other species previously assigned to Scolecostigmina belong in Pseudocercospora. Therefore, the verification of the proper generic affinity of S. curvispora needs phylogenetic data. Thus, we prefer to keep this species tentatively in Stigmina.
Stigmina knoxdaviesii Crous & U. Braun, Mycotaxon 57: 279 (1996).
(Fig. 28)
Fig. 28.
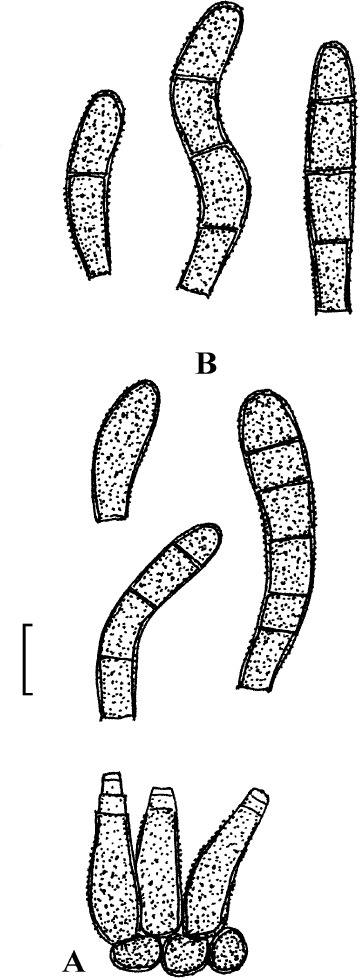
Stigmina knoxdaviesii (PREM 6618, lectotype). A. Part of a sporodochium. B. Conidia. Bar = 10 μm.
Replaced name: Cercospora caffra Syd. & P. Syd., Ann. Mycol. 12: 267 (1914), non Stigmina caffra (Wakef.) M.B. Ellis 1959.
Literature: Saccardo (1931: 868), Chupp (1954: 38), Crous & Braun (1996: 279), Crous & Braun (2003: 238).
Illustration: Crous & Braun (1996: 271, fig. 6).
Description: Leaf spots amphigenous, subcircular, 1–6 mm diam, brown, reddish brown to finally almost blackish, margin indefinite. Caespituli amphigenous, mainly hypophyllous, punctiform-pustulate, greyish brown, dark brown to blackish brown. Mycelium internal; hyphae sparingly branched, septate, brown, thin-walled, smooth. Stromata substomatal to immersed, large, subglobose to somewhat applanate, 50–85 μm wide and 20–30 μm high, pseudoparenchymatous, brown. Conidiophores numerous, in dense sporodochial fascicles, arising from stromata, through stomata or erumpent, erect, subcylindrical-conical, doliiform-ampulliform, unbranched, straight to slightly curved, aseptate, i.e. reduced to a single conidiogenous cell, 8–25 × 3–5 μm, pale olivaceous to brown, darker in mass, tips sometimes subhyaline, wall thin to slightly thickened, verruculose, proliferation percurrent, leaving 1–4 irregular annellations, terminal conidiogenous locus planate, unthickened, not darkened. Conidia solitary, cylindrical, subcylindrical to somewhat obclavate, straight to usually curved, (10–)15–45(–55) × 3.5–6 μm, (0–)3(–6)-septate, brown, wall thin to slightly thickened, verruculose, apex obtuse, base truncate to short obconically truncate, planate, hila unthickened and not darkened, occasionally with a minute frill.
Lectotype (designated here, MycoBank, MBT204894): South Africa: Transvaal: Nelspruit, Barberton, on Sclerocarya birrea subsp. caffra, May 1913, S. Hall (PREM 6618). Isolectotypes: CUP 39256, S-F23405.
Host range and distribution: On Sclerocarya birrea subsp. caffra [caffra], Anacardiaceae, South Africa (Transvaal).
Notes: This species is tentatively maintained in Stigmina s. lat. The latter genus has been reduced to synonymy with Pseudocercospora since the type species of Stigmina, S. platani (Fuckel) Sacc. (conidia distoseptate), clusters within the big Pseudocercospora clade (Crous et al. 2006), and Pseudocercospora has been conserved against the older name Stigmina (Braun & Crous 2006). However, the genuine generic affinity of S. knoxdaviesii is still unknown and not yet proven by means of sequence analyses. The shape and structure of the conidiophores, conidiogenous cells as well as euseptate conidia are reminiscent of Scolecostigmina, a genus segregated from Stigmina due to pluriseptate, scolecosporous conidia. The type species of the latter genus clusters distantly from the Pseudocercospora clade, but other species previously assigned to Scolecostigmina belong to Pseudocercospora (Crous et al. 2013). Therefore, a final conclusion about the generic affinity of S. knoxdaviesii requires phylogenetic data. The material deposited at PREM, which is in better condition than the meagre type collection in S, is designated as lectotype.
Stigmina pulviniformis (Syd.) S. Hughes, Mycol. Pap. 49: 24 (1952).
(Fig. 29)
Fig. 29.
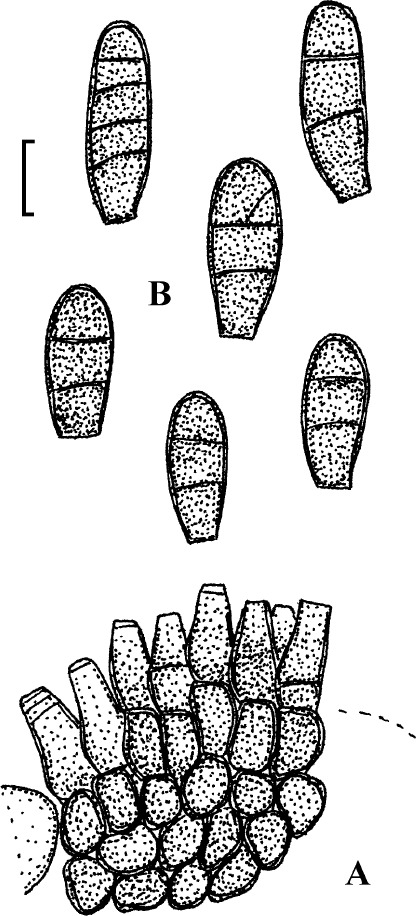
Stigmina pulviniformis (K(M) IMI 37988, isolectotype). A. Sporodochium. B. Conidia. Bar = 10 μm.
Basionym: Brachysporium pulviniforme Syd., Ann. Mycol. 12: 267 (1914).
Synonym: Sciniatosporium pulviniforme (Syd.) Morgan-Jones, Canad. J. Bot. 49: 1003 (1971).
Literature: Ellis (1976: 116), Crous & Braun (1996: 303).
Illustration: Ellis (1976: 115, fig. 82 C).
Description: Leaf spots lacking, indistinct. Caespituli hypophyllous, punctiform, in small groups, later confluent, forming continuous dark brown patches, pulvinate. Mycelium internal. Stromata large, immersed to somewhat erumpent, to 200 μm diam, brown. Conidiophores numerous, in large sporodochial conidiomata, arising from stromata, erumpent, subcylindrical to somewhat ampulliform, unbranched, apex more or less truncate, 10–35 × 5–9 μm, aseptate, with 0–4 conspicuous annellations. Conidia solitary, ellipsoid-ovoid to oblong, short subcylindrical, straight, rarely slightly curved, 18–35 × 8–11.5 μm, with 1–4 transverse septa and occasionally a single longitudinal or oblique septum, brown, wall somewhat thickened, smooth, apex rounded, base short obconically truncate, hila not thicker and not darker than the lateral wall.
Lectotype (designated here, MycoBank, MBT204895): South Africa: Cape Province: Cape Town, St James, on Rhus tomentosa, 22 Dec. 1912, G. B. Pole Evans 5575 (S-F21930). Isolectotypes: K(M) IMI 37988, PREM 5575.
Notes: Owing to the phylogenetic position of its type species, the genus Stigmina has been reduced to synonymy with Pseudocercospora. However, the phylogenetic affinity of S. pulviniformis is still unknown and this species is morphologically not scolecostigmina-like. On the other hand, it resembles the type species of Stigmina which proved to cluster within the Pseudocercospora clade. Stigmina pulviniformis needs to be sequenced and reassessed. Therefore, we prefer to maintain this species in Stigmina, at least tentatively until its phylogenetic affinity is clarified.
Stigmina rhois Crous & U. Braun, Sydowia 46: 221 (1994).
(Fig. 30)
Fig. 30.
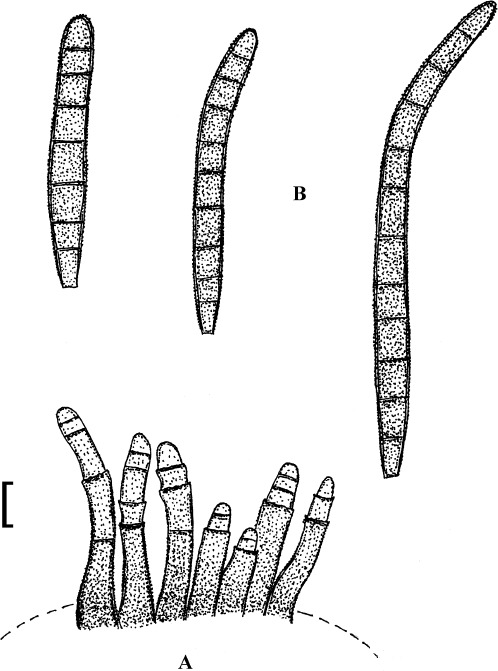
Stigmina rhois (PREM 51132, holotype). A. Part of a sporodochium with conidiophores. B. Conidia. Bar = 10 μm.
Illustration: Crous & Braun (1994: 222, fig. 11).
Description: Leaf spots epiphyllous, scattered, circular to irregular, 4–20 mm diam, dark brown. Caespituli epiphyllous, punctiform-pustulate, brown. Mycelium internal, composed of branched, septate, medium brown hyphae. Stromata well-developed, large, brown. Conidiophores numerous, in dense fascicles, arising from stromata, forming large sporodochia, 55–100 wide and 35–70 μm high, centered over stomata, dark brown, erect, straight, subcylindrical, somewhat geniculate-sinuous, unbranched, 30–50 × 5–8 μm, 1–2-septate, brown, thin-walled, verruculose; conidiogenous cells integrated, terminal, subcylindrical-ampulliform, proliferation percurrent, with to five distinct annellations. Conidia solitary, obclavate-subcylindrical, straight to curved, 45–130 × 6–8 μm, 3–19-septate, medium brown, thin-walled, verruculose, apex obtuse, rounded, base short obconically truncate, occasionally with a minute frill, hila unthickened, not darkened.
Holotype: South Africa: Natal: Mahai George, Royal Natal National Park, on Rhus discolor, Anacadiaceae, May 1988, R. Y. Anelich (PREM 51132).
Host range and distribution: Only known from the type collection.
Note: See notes under Stigmina curvispora.
Scolecostigmina
A single species.
Scolecostigmina mangiferae (Koord.) U. Braun & Mouch., New Zealand J. Bot. 37: 323 (1999).
(Fig. 31)
Fig. 31.
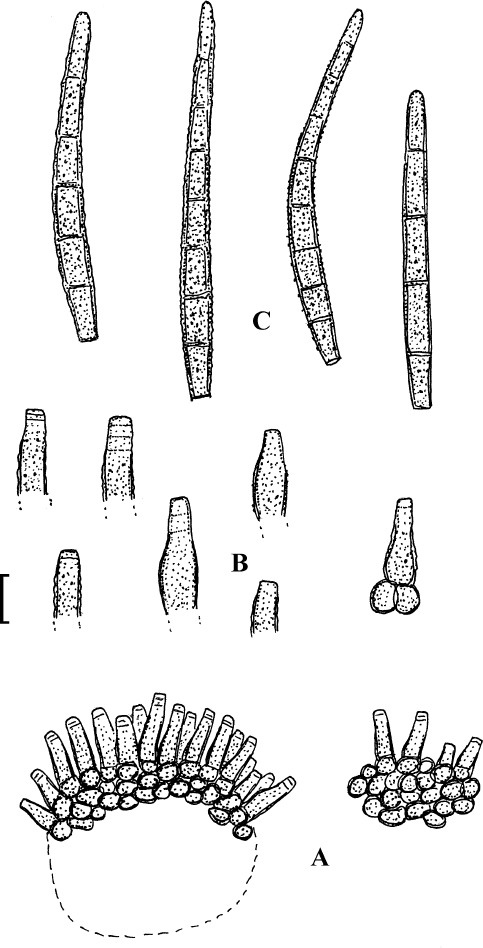
Scolecostigmina mangiferae (PC, Bugnicourt, NC 59.061a,b). A. Sporodochial conidiomata. B. Conidiophores. C. Conidia. Bar = 10 μm.
Basionym: Cercospora mangiferae Koord., Verh. Kon. Ned. Akad. Wetensch., Afd. Natuurk., Tweede Sect. 13: 236 (1907).
Synonyms: Stigmina mangiferae (Koord.) M.B. Ellis, Mycol. Pap. 72: 49 (1959).
Sciniatosporium mangiferae (Koord.) Morgan-Jones, Canad. J. Bot. 49: 999 (1971).
Literature: Saccardo (1913: 1414), Chupp (1954: 39–40), Ellis (1971: 146), Hsieh & Goh (1990: 21), Hyde (1992), Braun & Freire (2003: 325), Crous & Braun (2003: 265–266), Kamal (2010: 260), Phengsintham et al. (2013: 151–152), Crous et al. (2013: 74).
Illustrations: Ellis (1971: 145, fig. 97 C), Hyde (1992: 1, fig., unnumbered), Braun et al. (1999: 322, fig. 26), Phengsintham et al. (2013: 152, fig. 172, 153, fig. 173), Crous et al. (2013: 75, fig. 18).
Exsiccatae: Petr., Mycoth. Gen. 1210. Syd., Fungi Exot. Exs. 916.
Description: Leaf spots amphigenous, shape and size variable, subcircular to angular-irregular, 0.5–6 mm diam, occasionally larger, to 13 mm diam, dark brown to blackish, with yellowish, greenish to reddish brown margin or halo. Caespituli hypophyllous, punctiform, scattered, dark olivaceous brown to blackish. Mycelium internal. Stromata small to usually well-developed, (10–)15–70 μm diam, substomatal to immersed, often erumpent, dark brown, composed of swollen hyphal cells, circular to somewhat angular-irregular in outline, 3–7 μm diam. Conidiophores in small to usually large fascicles arising from stromata, dense, mostly sporodochial, emerging through stomata or erumpent, erect, straight or only slighty curved, subcylindrical to conical, ampulliform, unbranched, short, 5–25 × 3–5 μm, 0–1(–2)-septate, often aseptate, olivaceous brown to dark fuligineous, paler towards the tip, wall 0.5–1 μm wide, smooth or almost so to distinctly verruculose, apex truncate; conidiogenous cells integrated, terminal or often reduced to conidiogenous cells, 5–20 μm long, with a truncate tip, unilocal, about 2–3 μm wide, unthickened, not darkened, proliferation percurrent, with 1–7 conspicuous, dense annellations. Conidia solitary, obclavate, obclavate-cylindrical or broadly fusoid, straight to curved, 20–80 × 3–6 μm, 2–10-septate, usually euseptate, but occasionally mixed with few distosepta, dark olivaceous to medium dark brown, sometimes with a reddish tinge, wall slightly thickened, 0.3–0.8 μm, smooth to rugose-verruculose, apex obtuse, base short obconically truncate, occasionally long obconically truncate, 2–4 μm wide, hila neither thicker nor darker compared to the lateral walls, sometimes with minute frill.
Holotype: Indonesia: Java: Kedu, Purworedjo, on Mangifera indica, 21 Sep. 1905, S. H. Koorders 6 ser. 5 (type material not traced, probably not preserved).
Host range and distribution: On Mangifera (foetida, indica), Anacardiaceae, Africa (Angola, Congo, Gabon, Ghana, Malawi, Mozambique, Nigeria, São Tomé e Príncipe, Sierra Leone, Somalia, Sudan, Tanzania, Uganda, Zambia), Asia (Brunei, China, India, Indonesia, Japan, Laos, Malaysia, Nepal, Papua New Guinea, Philippines, Sri Lanka, Taiwan, Thailand), Australia, North America (Mexico; USA, Florida), Central and South America (Argentina, Brazil, Colombia, Honduras, Panama, Suriname, Venezuela), Oceania (Cook Islands, Fiji, New Caledonia, Samoa, Solomon Islands, Tonga, Vanuatu), West Indies (Cuba, Dominican Republ., Haiti, Jamaica, Puerto Rico, Trinidad and Tobago, Virgin Islands).
Notes: Braun et al. (1999) introduced the genus Scolectostigmina with Cercospora mangiferae as type species. This genus is morphologically close to Pseudocercospora, above all to species with percurrently proliferating conidiogenous cells, previously assigned to Cercostigmina, which is now a synonym of Pseudocercospora, but distinguished by having conidiogenous cells with coarse annellations, usually verruculose, and thick-walled conidia. The morphological differentiation from Pseudocercospora spp. is not sharp and rather gradual, but molecular analyses recently carried out by Crous et al. (2013) unequivocally confirmed Scolectostigmina as a genus of its own. Type material could not be traced. Suitable material for a neotypification from Java was also not available. Therefore, the typification of this species has been postponed and should be done in connection with fresh collections, cultures and sequence analyses.
Zasmidium s. lat. (zasmidium-like asexual morph)
A single species.
Cercospora chandleri Hansf., Proc. Linn. Soc. London 155: 55 (1943).
(Fig. 32)
Fig. 32.
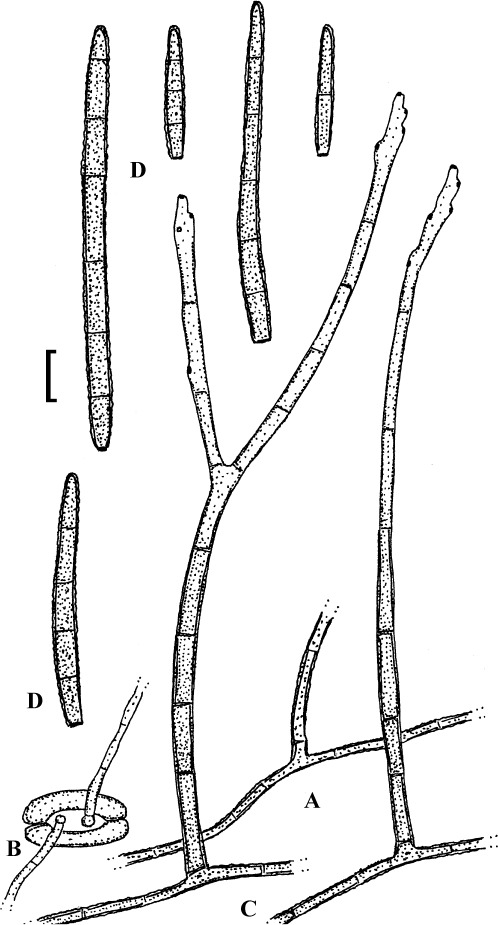
Cercospora chandleri (K(M) IMI 8594, holotype). A. Superficial hypha. B. Superficial hyphae emerging through a stoma. C. Solitary conidiophores arising from superficial hyphae. D. Conidia. Bar = 10 μm.
Synonym: Stenella chandleri (Hansf.) Suj. Singh & Kamal, Indian Phytopathol. 31: 316 (1979) [“1978”].
Literature: Chupp (1954: 39), Crous & Braun (2003: 118).
Description: Leaf spots lacking, fructification mixed with leaf hairs and mycelium of Asterina sp. Caespituli hypophyllous, effuse, olivaceous. Mycelium internal and external; superficial hyphae emerging through stomata, branched, mostly at right angles, straight to somewhat flexuous, sinuous, 1–3 μm wide, subhyaline, pale olivaceous to olivaceous brown, pale, thin-walled, verruculose, hyphae around conidiophores often somewhat darker, wider, to 4.5 μm, with slightly thickened walls. Stromata lacking. Conidiophores solitary, arising from superficial hyphae, on top of mother cells, erect, straight, cylindrical to filiform-setiform, rarely branched, only fertile upper part geniculate-sinuous or slightly nodulose, 100–300 × 3–5 μm, pluriseptate throughout, pale to medium dark brown throughout or somewhat paler towards the tip, wall slightly thickened, smooth to somewhat rough; conidiogenous cells integrated, terminal or intercalary, 10–25 μm long, conidiogenous loci conspicuous, somewhat thickened and darkened, 1.5–2 μm diam. Conidia solitary, cylindrical to slightly cylindrical-obclavate, straight to slightly curved, 20–120(–200) × 3–6 μm, 1–11-septate, pale brown or olivaceous brown, thin-walled, verruculose, apex obtuse, base truncate or slightly obconically truncate, 2–2.5 μm wide, hila somewhat thickened and darkened.
Holotype: Uganda: Kampala, on Pseudospondias microcarpa, Anacardiaceae, Oct. 1938, Hansford 2532 (K(M) IMI 8594).
Host range and distribution: Only known from the type collection.
Notes: This is a species with typically zasmidium-like morphology. The combination of this species into Stenella by Singh & Kamal (1979) was based on Indian material on Cassia fistula, which is, however, not conspecific and belongs to Stenella cassiae Abbasi & D.N. Shukla. Cercospora chandleri does not cause any lesions, but forms mixed infections with Asterina sp., but the two species are not associated, i.e., C. chandleri is not fungicolous. The mycelium of this species is only associated with green leaf tissue, so that C. chandleri has to be considered a plant parasite. However, a proper reassessment of the generic affinity of this species is not possible and must be based on cultures and sequence data. The zasmidium-like morphology is not sufficient and unreliable for a reallocation since zasmidioid asexual morphs are associated with cercosporoid species belonging to various mainly phylogenetically circumscribed genera (see introduction). Therefore, we prefer to keep this species in Cercospora, at least tentatively until corresponding sequence analyses are available.
Annonaceae
Cercospora
A single species.
Cercospora apii s. lat. (sensu Crous & Braun 2003).
Notes: Cercospora collections morphologically indistinguish-able from C. apii s. lat. are known from Annona squamosa and Cananga odorata (Crous & Braun 2003: 61, 99, as “C. canescens”). The identity of the collections concerned is unclear. C. apii s. lat. is a complicated complex of plurivorous and specialised taxa (Groenewald et al. 2013). Reliable identifications of such collections have to be based on cultures and molecular sequence alayses.
Passalora
Key to Passalora species on Annonaceae
1 Mycelium internal; conidiophores fasciculate, long and broad, 50–110 × 5–9 μm; conidia formed singly, 35–130 × 7–9 μm, 1–4-septate; on Miliusa sp. ........................................................................... P. annonacerarum
Mycelium internal and external, with solitary conidiophores or, if mycelium only internal, conidiophores much narrower, 2–5 μm wide; conidia formed in chains, only 2–4.5 μm wide ................................. 2
2 (1) Superficial hyphae with solitary conidiophores present (mycovellosiella-like) ................................................................ 3
Superficial hyphae and solitary conidiophores lacking; conidiophores fasciculate (phaeoramularia-like species) ......... 4
3 (2) Stromata 10–30 μm diam; conidiophores in fascicles as well as solitary; conidia 25–80 μm long, 3–8-septate; on Annona sp. ............................................................................................................... P. annonigena
Stromata lacking; conidiophores consistently solitary, fascicles not formed; conidia 15–50 μm long, 2–4-septate; on Miliusa sp. ...................................................................................................................... P. miliusae
4 (2) Stromata poorly developed; conidiophores 100–200(–250) μm long; conidia subhyaline to pale brownish; on Isolona cauliflora ................................................................................................................................ P. isolonae
Stromata 10–30 μm diam; conidiophores 30–150 μm long; conidia hyaline or subhyaline; on Annona and Xylopia ............................................................................................................................ P. xylopiae
Tabular key to Passalora species on Annonaceae according to host genera
Annona
1 Mycelium internal and external; superficial hyphae with solitary conidiophores developed; conidiophores 10–60 μm long; conidia solitary, 25–80 × 3–6 μm, 3–8-septate, subhyaline, pale olivaceous to olivaceous brown .............................................................................. P. annonigena
Mycelium internal; superficial hyphae with solitary conidiophores not developed; conidiophores much longer, 30–150 μm; conidia at least partly in chains, 25–50(–65) × 2–4.5 μm, hyaline or subhyaline .............................................................................................................................. P. xylopiae
Isolona
A single species ........................................................................................................................................................... P. isolonae
Miliusa
1 Stromata lacking; mycelium internal and external; conidiophores solitary, arising from superficial hyphae, short, about 10–25 μm long; conidia 15–50 μm long and about 5 μm wide ............................................ P. miliusae
Stromata developed, 10–30 μm diam; superficial hyphae and solitary conidiophores absent; conidiophores fasciculate, much longer, 50–110 μm; conidia 35–130 μm long and 7–9 μm wide ...................................................................................................................................................... P. annonacearum
Xylopia
A single species ........................................................................................................................................................... P. xylopiae
Passalora species on Annonaceae
Passalora annonacearum A.N. Rai & Kamal, Kavaka 14: 33 (1987) [“1986”]; as“anonacearum“.
(Fig. 33)
Fig. 33.
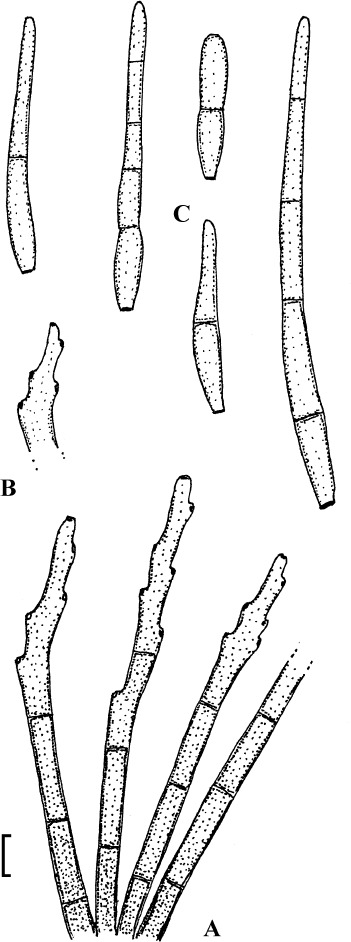
Passalora annonacearum (K(M) IMI 247367, holotype). A. Conidiophore fascicle. B. Conidiophore tip. C. Conidia. Bar = 10 μm.
Literature: Crous & Braun (2003: 438), Kamal (2010: 103).
Illustrations: Rai & Kamal (1987: 34, fig. 2)
Description: Leaf spots hypophyllous, greyish brown, confluent, covering large leaf segments. Caespituli hypophyllous, effuse, greyish brown, velvety. Mycelium internal; hyphae branched, septate, pigmented, thin-walled, smooth. Stromata relatively small, immersed, substomatal, subcircular to irregular in outline, 10–30 μm diam, medium to dark olivaceous brown. Conidiophores solitary or in small fascicles, divergent, erect, subcylindrical to distinctly geniculate-sinuous, unbranched, 50–110 × 5–9 μm, pluriseptate, pale to medium olivaceous or olivaceous brown, thin-walled, smooth; conidiogenous cells integrated, terminal, proliferation sympodial, with conspicuous conidiogenous loci, slightly thickened and darkened. Conidia solitary, cylindrical, subcylindrical to obclavate-cylindrical, straight to somewhat curved, 35–130 × 7–9 μm, 1–4-septate, subhyaline to pale olivaceous, thin-walled, smooth, apex subacute to obtuse, base obconically truncate, hila slightly thickened and darkened.
Holotype: India: Uttar Pradesh: West Baharaich Forest Division, Katarniaghat, on Miliusa sp., Annonaceae, Dec. 1979, A. N. Rai KR 399 (K(M) IMI 247367).
Host range and distribution: Only known from the type collection.
Passalora annonigena U. Braun & F. Freire, Cryptog., Mycol. 23: 297 (2003) [“2002”].
(Fig. 34)
Fig. 34.
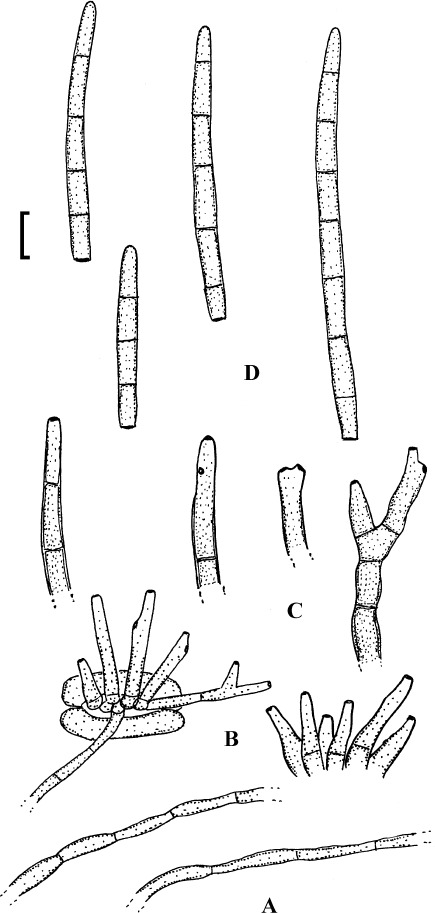
Passalora annonigena (HAL 1701 F, holotype). A. Superficial hyphae. B. Conidiophore fascicles. C. Conidiophores. D. Conidia. Bar = 10 μm.
Literature: Crous & Braun (2003: 438), Braun & Crous (2008).
Illustrations: Braun & Freire (2003: 298, fig. 2).
Description: Leaf spots amphigenous, angular-irregular, 2–20 mm diam, dark brown to blackish, later dingy greyish brown, margin indefinite or with a narrow darker border or marginal line, sometimes with a pale yellowish to ochraceous halo. Caespituli hypophyllous, punctiform, blackish brown. Mycelium internal and external; superficial hyphae sparingly branched, septate, 1.5–4 μm wide, pale olivaceous, smooth or almost so. Stromata substomatal, 10–30 μm diam, brown. Conidiophores in small to moderately large fascicles, loose to moderately dense, arising from stromata, through stomata, occasionally arising from superficial hyphae, lateral and occasionally terminal, erect, straight, subcylindrical to slightly geniculate-sinuous, unbranched or only rarely branched, 10–60 × 3–5 μm, continuous to pluriseptate throughout, pale olivaceous to reddish brown, tips paler, thin-walled to somewhat thickened, smooth; conidiogenous cells integrated, terminal, 10–20 μm long, conidiogenous loci conspicuous, somewhat thickened and darkened, 1.5–2 μm diam. Conidia solitary, cylindrical to cylindrical-obclavate, more or less straight, 25–80 × 3–6 μm, 3–8-septate, occasionally constricted at the septa, subhyaline, pale olivaceous to olivaceous brown, thin-walled, smooth, apex obtuse, base short obconically truncate, 1.5–2.5 μm diam, hila slightly thickened and darkened.
Holotype: Brazil: State of Ceará: Cascavel county, Preaoca district, on Annona sp., Annonaceae, 5 Sep. 1999, F. C. O. Freire (HAL 1701 F).
Host range and distribution: Only known from the type collection.
Passalora isolonae (G. Siboe et al.) U. Braun & Crous, Mycosphaerella Anam. 1: 457 (2003).
(Fig. 35)
Fig. 35.
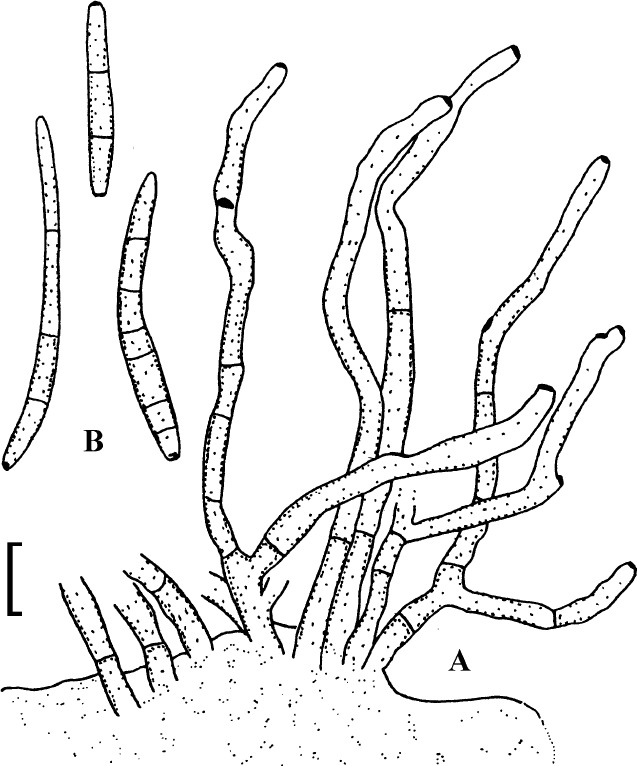
Passalora isolonae (based on Siboe et al. 2000: 300, fig. 6). A. Loose conidiophore fascicle. B. Conidia. Bar = 10 μm.
Basionym: Phaeoramularia isolonae G. Siboe et al., Sydowia 52: 299 (2000).
Illustrations: Siboe et al. (2000: 300, fig. 6)
Description: Leaf spots more or less circular, poorly differentiated, dark brown to greyish. Caespituli amphigenous, effuse, greyish to olivaceous brown. Stromata poorly developed. Hyphae internal. Conidiophores in fascicles, loose to rather dense, erect, subcylindrical-filiform, geniculate-sinuous, simple or branched, 100–200(–250) × 2.5–4 μm, 2–5-septate, brown, thin-walled, smooth to faintly verruculose; conidiogenous cells integrated, terminal, 15–60 μm long, proliferation sympodial, conidiogenous loci conspicuous, thickened and darkened, about 1.5–2 μm wide. Conidia solitary and in short chains, cylindrical or subcylindrical, straight to somewhat curved, 30–70 × 3–4 μm, 2–7-septate, subhyaline to pale brownish, thin-walled, smooth, apex obtuse to truncate in catenate conidia, base short obconically truncate, about 1.5–2 μm wide, hila slightly thickened and darkened.
Holotype: Kenya: Kwale, Shimba Hills National Park, Makadara Forest, on Isolona cauliflora, Annonaceae, 21 Sep. 1999, G. M. Siboe (NAI (M) 4384).
Host range and distribution: Only known from the type collection.
Passalora miliusae U. Braun & Crous, Mycosphaerella Anam. 1: 460 (2003).
(Fig. 36)
Fig. 36.
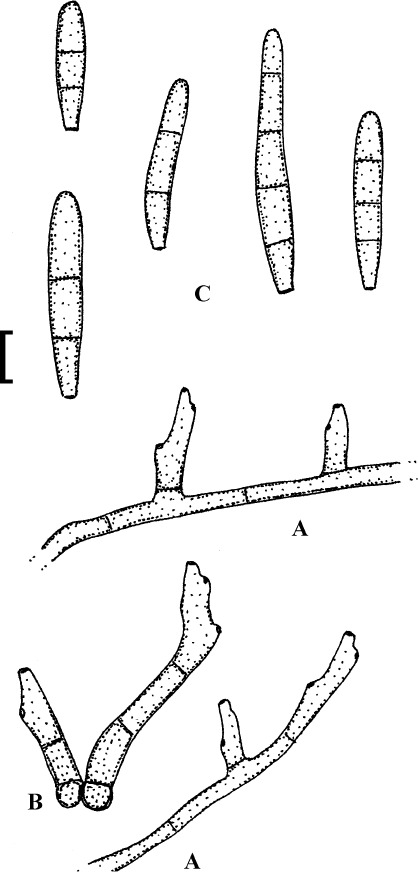
Passalora miliusae (K(M) IMI 228205, holotype). A. Solitary conidiophores arising from superficial hyphae. B. Small conidiophore fascicle. C. Conidia. Bar = 10 μm.
Basionym: Mycovellosiella indica P. Kumar & Kamal, Curr. Sci. 51: 846 (1982), non Passalora indica Kamal & P. Kumar 1981.
Literature: Kamal (2010: 129)
Illustrations: Kumar & Kamal (1982: 846, figs 1–2).
Description: Leaf spots indistinct. Colonies hypophyllous, effuse. Mycelium internal and external; superficial hyphae emerging through stomata, branched, septate, to 3 μm wide, subhyaline, thin-walled, smooth. Stromata absent. Conidiophores solitary, arising from superficial hyphae, lateral and terminal, erect, straight, subcylindrical to distinctly geniculate-sinuous, simple or branched, about 10–25 μm long and 3–4 μm wide, 0–2-septate; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, sympodial, with conspicuous conidiogenous loci, thickened and darkened. Conidia solitary, cylindrical, subcylindrical to somewhat clavate, more or less straight, 15–50 μm long, about 5 μm wide, 2–4-septate, subhyaline, apex rounded, base obconically truncate, about 2 μm wide, hila somewhat thickened and darkened.
Holotype: India: Uttar Pradesh: Gorakhpur, on Miliusa tomentosa, Annonaceae, Feb. 1978, P. Kumar 262 (K(M) IMI 228205).
Host range and distribution: Only known from the type collection.
Passalora xylopiae (Viégas & Chupp) U. Braun & Crous, Mycosphaerella Anam. 1: 432 (2003).
(Fig. 37)
Fig. 37.
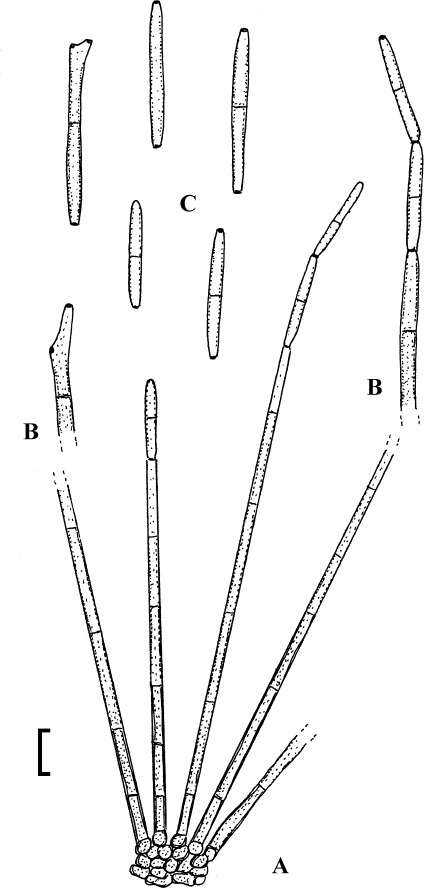
Passalora xylopiae (CUP 41581, lectotype). A. Conidiophore fascicle. B. Conidiophore tips. C. Conidia. Bar = 10 μm.
Basionym: Cercospora xylopiae Viégas & Chupp, Bol. Soc. Brasil. Agron. 8: 58 (1945).
Literature: Chupp (1954: 46).
Description: Leaf spots amphigenous, subcircular to angular-irregular, 2–8 mm diam, sometimes oblong between veins, dark brown, later greyish brown, with a narrow darker border. Caespituli amphigenous, finely punctiform to subeffuse. Mycelium internal. Stromata small, about 10–30 μm diam, brown, substomatal to immersed. Conidiophores in loose fascicles, about 2–15, arising from stromata, through stomata or erumpent, erect, straight, subcylindrical-filiform, not or barely geniculate, unbranched, 30–150 × 2–5 μm, pluriseptate throughout, subhyaline to pale olivaceous or olivaceous brown below, paler towards the apex, tips hyaline or subhyaline, thin-walled, smooth; conidiogenous cells integrated, terminal, 10–25 μm long, conidiogenous loci conspicuous, (1–)1.5–2 μm wide, slightly thickened and darkened. Conidia in simple, occasionally branched chains, cylindrical or subcylindrical, more or less straight, 20–50(–65) × 2–4.5 μm, 0–5-septate, hyaline or subhyaline, thin-walled, smooth, apex obtuse to truncate, base truncate to short obconically truncate, 1.5–2 μm wide, hila slightly thickened and darkened.
Lectotype (designated here, MycoBank MBT204896): Brazil: São Paulo, Ribeirao Preto, Experiment Station, on Xylopia aromatica, 30 May 1935, A. S. Costa & H. P. Krug 690 (CUP 41581). Isolectotype: IACM 690.
Host range and distribution: On Annona (dioica, muricata), Xylopia aromatica [grandiflora], Annonaceae, South America (Brazil, Dominican Republic).
Notes: The conidiophores are filiform with mostly unilocal conidiogenous cells and conspicuous conidiogenous loci. The conidia are catenate, occasionally in branched chains, i.e. this species is morphologically phaeoramularioid. Detached conidiophores or conidiophore fragments might be confused with longer conidia. The record from the Dominican Republic on Annona muricata refers to a collection deposited as BPI 442561.
Key to Pseudocercospora species on Annonaceae
1 Conidiophores in synnematous conidiomata; on Annona squamosa ........................................................P. annonarum
Conidiophores solitary or fasciculate, at most in sporodochial conidiomata, but not synnematous ............................... 2
2 (1) Superficial mycelium in vivo present; conidiophores solitary as well as fasciculate, sometimes even in sporodochial conidiomata ......................................................................................................... 3
Superficial mycelium in vivo not developed, mycelium consistently internal ................................................................... 8
3 (2) Conidia very broad, 20–80 × 4–12 μm, with 1–9 transverse and occasionally 1(–2) oblique or longitudinal septa; on Asimina spp. .................................................................................................... P. asiminae
Conidia much narrower, to 7 μm, only with transverse septa ......................................................................................... 4
4 (3) Conidiophores consistently solitary, arising from superficial hyphae, distinct fascicles not formed, 12–70 × 3.5–5 μm; conidia narrowly obclavate-cylindrical, 40–156 × 2.5–3.5 μm, 3–13-septate; on Polyalthia suberosa, India ............................................................................................................... P. polyalthiae
Conidiophores solitary as well as fasciculate .................................................................................................................. 5
5 (4) Conidia very long and wide, 50–150 × 5–7 μm, densely pluriseptate (6–18), hila 2.5–3.5 μm wide; conidiophores usually fasciculate, occasionally solitary, arising from superficial hyphae; on Annona spp., Asia, Central and South America, West Indies ............................................................. P. annonae
Conidia narrower, 1.5–6(–7) μm wide, with to 10 septa, hila narrower, 1–2.5 μm .......................................................... 6
6 (5) Conidia 25–150 × 3–6(–7) μm, very pale, mostly subhyaline; on Asimina spp., North America .............................................................................................................................................. P. asiminae-pygmaeae
Conidia narrower, 1.5–4 μm, subhyaline to pale brown; on Annona and Rollinia spp. .................................................... 7
7 (6) Superficial mycelium with solitary conidiophores abundant, fascicles of conidiophores variable, small and loose to large and dense, occasionally in almost sporodochial conidiomata; apex of conidia obtuse in cylindrical conidia to subacute in obclavate conidia; on Annona spp. and Rollinia mucosa, Asia, West Indies, Central and South America .................................. P. annonae-squamosae
Mycelium internal and external, but conidiophores in dense fascicles, solitary conidiophores not formed; apex of conidia obtuse; on Annona purpurea, Brazil ............................................... P. xenoannonicola
8 (2) Conidiophores in sporodochial conidiomata; conidiogenous cells monoblastic, percurrent, with distinct annellations; conidia rough-walled ....................................................................................................... 9
Conidiogenous cells monoblastic to usually polyblastic, sympodial, annellations lacking; conidia smooth or almost so ................................................................................................................................. 10
9 (8) Conidiophores 30–50 μm long, verruculose; conidia 40–120 × 4.5–8 μm, obclavate-cylindrical; on Annona senegalensis, South Africa ...................................................................................................... P. oblecta
Conidiophores 5–20(–30) μm long, smooth; conidia narrower, 20–220 × 3.5–5 μm, cylindrical-vermiform; on Annona crassifolia, Brazil ............................................................................. P. annonifolii
10 (8) Conidia broad, 5–8 μm; conidiophores in small, more or less loose fascicles ............................................................... 11
Conidia narrower, 2–6 μm; conidiophores usually numerous, in more or less dense, often sporodochial fascicles or, if not sporodochial, conidia very narrow, only 2–3 μm wide ................................12
11 (10) Conidiophores fasciculate, 60–220 μm long; conidia obclavate, 50–110 μm long, base obconically truncate; on Annona senegalensis and Annona sp., Africa, Asia .................................... P. scitula
Conidiophores much shorter, to 100 μm long, fasciculate, occasionally solitary, arising from superficial hyphae; conidia broadly acicular-obclavate, base truncate to slightly obconically truncate; on Annona spp. and Rollinia spp. ................................... P. annonae
12 (10) Conidia subcylindrical-filiform to subacicular, 50–115 × 3.5–6 μm, hyaline to pale olivaceous; on Miliusa sp., India .............................................................................................................................. P. annonacea
Conidia obclavate-cylindrical, narrower, 2–3 μm wide, pale olivaceous to olivaceous brown ...................................... 13
13 (12) Conidia (30–)50–80(–100) μm long; mycelium internal and external; on Annona montana, Brazil .................................................................................................................................................... P. xenoannonicola
Conidia shorter, 20–65 μm; mycelium consistently internal ............................................................................................ 14
14 (13) Leaf spots to 1 mm diam, brown, later greyish with brown margin; stromata small, 10–25 μm diam; conidiophores very pale olivaceous; conidia 3–7-septate; on Xylopia aethiopica, Africa .................... P. aethiopicae
Leaf spots 1–21 mm diam; brown to blackish; stromata larger, 15–40 μm diam; conidiophores light brown; conidia 0–4-septate; on Miliusa tomentosa, Asia (India) ........... P. miliusae-tomentosae
Tabular key to Pseudocercospora species on Annonaceae according to host genera
Annona
1 Conidiophores in synnematous conidiomata; on Annona squamosa ........................................................ P. annonarum
Conidiophores solitary or fasciculate, at most in sporodochial conidiomata, but not synnematous ............................... 2
2 (1) Superficial mycelium in vivo developed; conidiophores solitary as well as fasciculate, sometimes even in sporodochial conidiomata .......................................................................................................... 3
Superficial mycelium in vivo not developed, mycelium consistently internal ................................................................... 5
3 (2) Conidia very long and wide, 50–150 × 5–7 μm, densely pluriseptate (6–18), hila 2.5–3.5 μm wide; conidiophores usually fasciculate, occasionally solitary, arising from superficial hyphae; on Annona spp., Asia, Central and South America, West Indies ............................................................. P. annonae
Conidia narrower, 1.5–4 μm wide, with to 8 septa, hila narrower, 1–2 μm ...................................................................... 4
4 (3) Superficial mycelium with solitary conidiophores abundant, fascicles of conidiophores variable, small and loose to large and dense, occasionally in almost sporodochial conidiomata; apex of conidia obtuse in cylindrical conidia to subacute in obclavate conidia; on Annona spp. and Rollinia mucosa, Asia, West Indies, Central and South America ................................. P. annonae-squamosae
Mycelium internal and external, but conidiophores in dense fascicles, solitary conidiophores not formed; apex of conidia obtuse; on Annona purpurea, Brazil .................................................................. P. xenoannonicola
5 (2) Conidiophores in sporodochial conidiomata; conidiogenous cells monoblastic, percurrent, with distinct annellations; conidia rough-walled ........................................................................................................ 6
Conidiogenous cells monoblastic to usually polyblastic, sympodial, annellations lacking; conidia smooth or almost so ................................................................................................................................... 7
6 (5) Conidiophores 30–50 μm long, verruculose; conidia 40–120 × 4.5–8 μm, obclavate-cylindrical; on Annona senegalensis, South Africa ....................................................................................................... P. oblecta
Conidiophores 5–20(–30) μm long, smooth; conidia narrower, 20–220 × 3.5–5 μm, cylindrical-vermiform; on Annona crassifolia, Brazil ............................................................................. P. annonifolii
7 (5) Conidia (30–)50–80(–100) × 2–3 μm; mycelium internal and external, but without solitary conidiophores; on Annona montana, Brazil ......................................................................................................... P. xenoannonicola
Conidia much broader, 5–8 μm; mycelium consistently internal ...................................................................................... 8
8 (7) Conidiophores fasciculate, very long, 60–220 μm; conidia obclavate, 50–110 μm long, base obconically truncate; on Annona senegalensis and Annona sp., Africa, Asia .................................... P. scitula
Conidiophores much shorter, to 100 μm long, fasciculate, occasionally solitary, arising from superficial hyphae; conidia broadly acicular-obclavate, base truncate to slightly obconically truncate; on Annona spp. and Rollinia spp. ................................... P. annonae
Asimina
1 Conidia 25–150 × 3–6(–7) μm, transversely septate .................................................................. P. asiminae-pygmaeae
Conidia very broad, 20–80 × 4–12 μm, with 1–9 transverse and occasionally 1(–2) oblique or longitudinal septa ................................................................................................................................... P. asiminae
Miliusa
1 Stromata 30–75 μm diam; conidiophores pale olivaceous; conidia subcylindrical-filiform or only slightly obclavate-cylindrical, 50–115 × 3.5–6 μm, 4–17-septate, hyaline to pale olivaceous .................................................................................................................... P. annonacea
Stromata smaller, 15–40 μm diam; conidiophores light brown; conidia much shorter and narrower, 20–60 × 2–3 μm, 0–4-septate, pale brown ......................................................................... P. miliusae-tomentosae
Polyanthia
A single species ...................................................................................................................................................... P. polyalthiae
Rollinia
1 Conidia 50–150 × 5–7 μm, 6–18-septate ........................................................................................................ P. annonae
Conidia (15–)20–75(–80) × (1.5–)2–4(–5) μm, (1–)2–6(–8)-septate ......................................... P. annonae-squamosae
Xylopia
A single species ...................................................................................................................................................... P. aethiopicae
Pseudocercospora species on Annonaceae
Pseudocercospora aethiopicae Deighton, Mycol. Pap. 140: 13 (1976).
(Fig. 38)
Fig. 38.
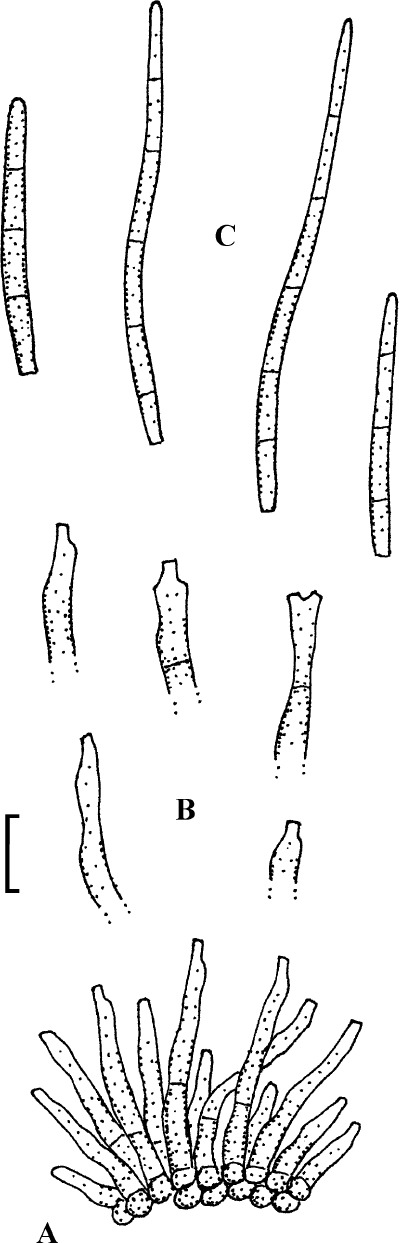
Pseudocercospora aethiopicae (K(M) IMI 7717, holotype). A. Conidiophore fascicle. B. Conidiophores. C. Conidia. Bar = 10 μm.
Literature: Braun & Crous (2008).
Illustration: Deighton (1976: 14, fig. 3).
Description: Leaf spots at first brown, more or less angular, to 1 mm diam, later greyish with a brown margin, irregularly angular, to 5 mm diam, often confluent. Caespituli mostly hypophyllous, minutely punctiform, pale olivaceous. Mycelium internal; hyphae branched, septate, almost colourless, 1–2 μm wide. Stromata substomatal, about 25 μm diam and 10 μm high, olivaceous brown. Conidiophores in small to moderately large fascicles, mostly relatively dense, straight, subcylindrical to somewhat geniculate-sinuous, unbranched, 10–40 × 2.5–4 μm, 0–2-septate, very pale olivaceous, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, 10–25 μm long, conidiogenous loci inconspicuous or subdenticulate, 1–1.5 μm diam, but always unthickened and not darkened. Conidia solitary, obclavate-cylindrical, straight to curved, occasionally sigmoid, 30–65 × 2.5–3 μm, 3–7-septate, very pale olivaceous, thin-walled, smooth, apex obtuse, base short obconically truncate, 1–2 μm wide, hila unthickened and not darkened.
Holotype: Sierra Leone: Njala (Kori), on Xylopia aethiopica, 28 Jan. 1934, F. C. Deighton (K(M) IMI 7717).
Host range and distribution: On Xylopia aethiopica, Annonaceae, Africa (Sierra Leone).
Pseudocercospora annonacea (Kamal et al.) U. Braun, Nova Hedwigia 58: 209 (1994).
(Fig. 39)
Fig. 39.
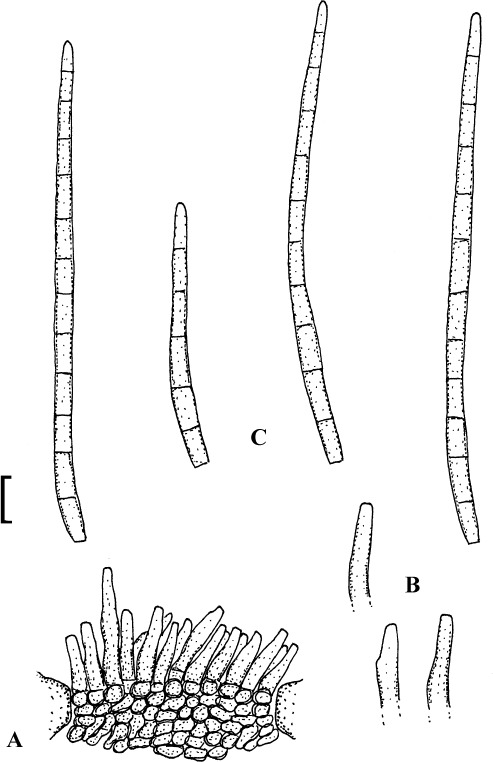
Pseudocercospora annonacea (K(M) IMI 283994, isotype). A. Conidiophore fascicle. B. Conidiophores. C. Conidia. Bar = 10 μm.
Basionym: Cercoseptoria annonacea Kamal et al., Indian Phytopathol. 39: 455 “1986” (1987); as “anonacea”.
Synonyms: Pseudocercosporella miliusae M.D. Mehrotra & R.K. Verma, Mycol. Res. 95: 1168 (1991) [holotype: India: Uttarakhand: Dehra Dun, Lacchiwala, on Miliusa velutina, without date, M. D. Mehrotra (FRIDD-36)].
Literature: Braun & Crous (2008: 218), Kamal (2010: 149).
Illustrations: Kamal et al. (1987: 455, fig. 2), Mehrotra & Verma (1991: 1168, fig. 27).
Description: Leaf spots amphigenous, subcircular to angular-irregular, 3–10 mm diam, sometimes confluent and larger, greyish to dark brown, margin yellowish brown. Caespituli amphigenous, punctiform, scattered, floccose, dark brown. Mycelium internal. Stromata large, 30–75 μm diam and 10–15 μm high, substomatal to immersed, olivaceous brown. Conidiophores numerous, in large, dense, sporodochial fascicles, arising from stromata, through stomata or erumpent, erect, straight, subcylindrical or somewhat attenuated towards the tip, slightly geniculate-sinuous, unbranched, 10–50 × 2.5–4.5 μm, 0–2-septate, pale olivaceous, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores mostly reduced to conidiogenous cells, 10–30 μm long, conidiogenous loci inconspicuous. Conidia solitary, subcylindrical-filiform to slightly obclavate-cylindrical, straight to curved, 50–115 × 3.5–6 μm, 4–17-septate, hyaline to pale olivaceous, thin-walled, smooth, apex obtuse, base short obconically truncate, hila unthickened, not darkened.
Holotype: India: Uttar Pradesh: North Gonda Forest Division, on Miliusa velutina, Jan. 1984, P. Narayana (GPU 94). Isotype: K(M) IMI 283994.
Host range and distribution: On Miliusa velutina, Annonaceae, Asia (India, Uttarakhand, Uttar Pradesh).
Pseudocercospora annonae U. Braun & Crous, Feddes Repert. 113: 117 (2002).
(Fig. 40)
Fig. 40.
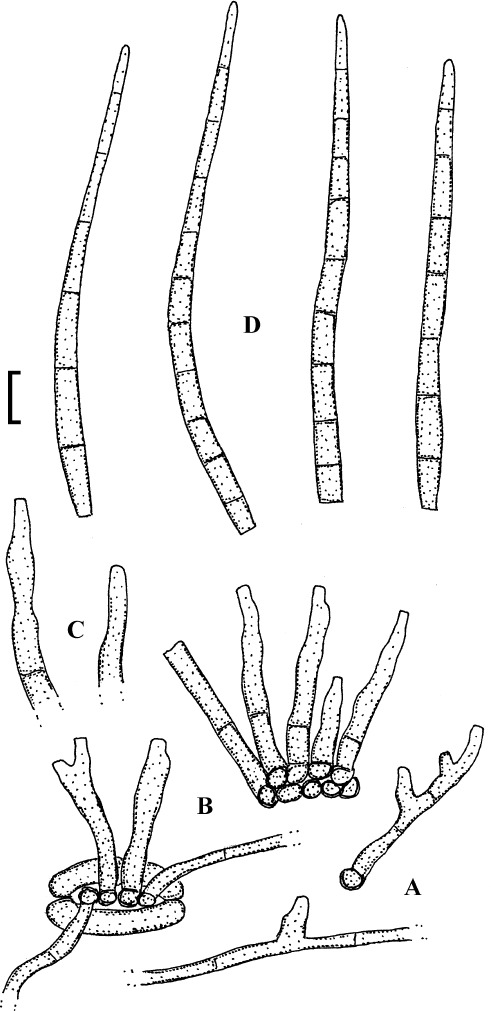
Pseudocercospora annonae (CUP-MG-000335, holotype). A. Solitary conidiophores arising from superficial hyphae. B. Conidiophore fascicle. C. Conidiophore tips. D. Conidia. Bar = 10 μm.
Synonym: Cercospora annonae A.S. Mull. & Chupp, Arq. Inst. Biol. Veg., Rio de Janeiro 1: 214 (1935); as “anonae”, nom. inval. (Art. 39.1) [holotype: Brazil: Minas Gerais: Viçosa-Escola, on Annona squamosa, 7 Apr. 1932, A. S. Muller 335 (CUP-MG-000335)].
Literature: Chupp (1945: 45), Vasudeva (1963: 39), Crous & Braun (2003: 59), Braun & Crous (2008: 218), Kamal (2010: 149).
Illustrations: Braun et al. (2002: 115, fig. 5).
Description: Leaf spots amphigenous, angular-irregular, 0.5–5 mm diam, often vein-limited, blackish, margin indefinite. Caespituli amphigenous, punctiform to subeffuse, brown. Mycelium internal and external; superficial hyphae lacking to well-developed, emerging through stomata, sparingly branched, septate, 1.5–4 μm wide, subhyaline to pale olivaceous, thin-walled, smooth. Stromata lacking or small, composed of a few swollen hyphal cells, substomatal, brown. Conidiophores in small to moderately large fascicles, loose to dense, arising from internal hyphae or stromatic hyphal aggregations or arising from superficial hyphae, lateral and occasionally terminal, erect, straight, subcylindrical, flexuous, simple or rarely branched, barely geniculate-sinuous, 10–110 × 3–5 μm, 0–2-septate, pale olivaceous, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, 10–30 μm long, conidiogenous loci inconspicuous. Conidia solitary, obclavate-cylindrical, broadly subacicular, 50–150 × 5–7 μm, densely pluriseptate, usually 6–18-septate, distance between septa 4–15 μm, subhyaline to medium olivaceous, thin-walled, smooth, apex subobtuse to subacute, base truncate to somewhat obconically truncate, 2.5–3.5 μm wide, hila unthickened, not darkened.
Holotype: Brazil: Minas Gerais: Viçosa-Escola, on Annona squamosa, 7 Apr. 1932, A. S. Muller 335 (CUP-MG-000335).
Host range and distribution: On Annona (cherimolia, muricata, reticulata, squamosa, Annona sp.), Rollinia sylvatica, Annonaceae, Asia (India, Andhra Pradesh; Philippines), Central and South America (Brazil, Guatemala, Panama, Venezuela), West Indies (Cuba).
Notes: This species is common on Annona spp. The single record on Rollinia sylvatica refers to a collection deposited as CUP-MG-000779 (Brazil, Minas Gerais, Viçosa-Escola, 29 Apr. 1934, A.S. Muller 779).
Pseudocercospora annonae-squamosae U. Braun & R.F. Castañeda, Cryptog. Bot. 1: 50 (1989).
(Fig. 41)
Fig. 41.
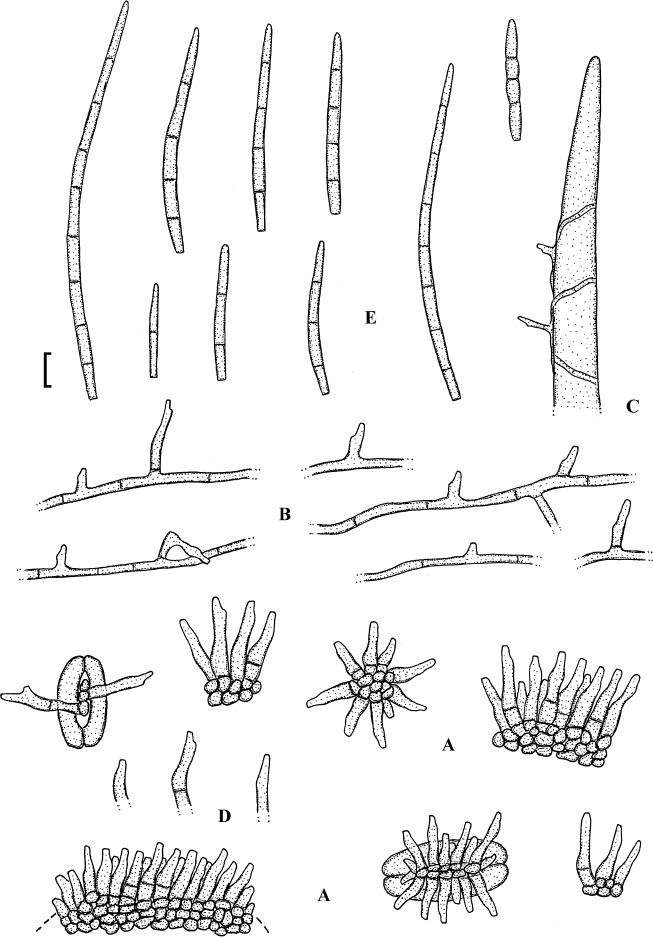
Pseudocercospora annonae-squamosae (HAL 1650 F, holotype). A. Conidiophore fascicles. B. Solitary conidiophores arising from superficial hyphae. C. Superficial hyphae climbing a leaf hair. D. Conidiophores. E. Conidia. Bar = 10 μm.
Synonyms: Cercospora caracasensis Chupp & A.S. Mull., Bol. Soc. Venez. Ci. Nat. 8: 39 (1942), nom. inval. (Art. 39.1) [holotype: Venezuela: Caracas, on Annona purpurea, 1 Mar. 1938, A. S. Muller (CUP-VZ 2111); isotype: VIA 2111].
Pseudocercospora annonicola Goh & W.H. Hsieh, Cercospora and similar fungi from Taiwan: 22 (1999) [holotype: Taiwan: Taitung, on Annona squamosa, 24 Mar. 1987, T. K. Goh (NCHUPP-152d)].
Literature: Chupp (1954: 45), Vasudeva (1963: 67), Guo & Hsieh (1995: 16), Guo et al. (1998: 27–28), Nakashima et al. (2002: 96), Crous & Braun (2003: 59), Braun & Freire (2004: 226), Braun & Crous (2008: 218–220), Kamal (2010: 149).
Illustrations: Castañeda Ruiz & Braun (1989: 48, plate 19, fig. 4), Hsieh & Goh (1990: 22, fig. 10), Guo & Hsieh (1995: 16), fig. 15), Guo et al. (1998: 28, fig. 16), Nakashima et al. (2002: 98, fig. 3), Braun & Crous (2008: 219, fig. 4).
Exsiccatae: U. Braun, Fungi Sel. Exs. 9.
Description: Leaf spots amphigenous, subcircular to angular-irregular, 1–30 mm diam, occasionally confluent, reddish brown, medium to dark brown or sometimes even blackish brown, later often greyish brown to dingy grey, finally greyish white, often vein-limited, margin indefinite or with darker narrow margin or marginal line, brown. Caespituli amphigenous, on the upper side conspicuous, punctiform, scattered, dark brown to blackish, but soon turning greyish by abundant conidial formation, on the lower leaf surface slightly effuse and less conspicuous, dingy greyish olivaceous to olivaceous brown. Mycelium internal and external, superficial hyphae variable, ranging from almost lacking to well-developed, usually hypophyllous, emerging through stomata, occasionally climbing leaf hairs, branched, septate, 1–3.5 μm wide, pale olivaceous to olivaceous brown, thin-walled, smooth. Stromata almost absent to well-developed, above all on the upper side, 10–80 μm diam, substomatal to intraepidermal, olivaceous brown. Conidiophores in small, loose to large, dense fascicles, often almost sporodochial on the upper side, arising from internal hyphae or from stromata and solitary, arising from superficial hyphae (if present, and mainly on the lower side), lateral, rarely terminal, erect, straight, subcylindrical, conical to distinctly geniculate-sinuous, usually unbranched, 5–30(–45) × 2–4(–5) μm, 0–1(–2)-septate, subhyaline to pale olivaceous or olivaceous brown, medium olivaceous brown in mass, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, 5–20 μm long, conidiogenous loci inconspicuous to subconspicuous by being truncate or subdenticulate, but always unthickened and not darkened. Conidia solitary, obclavate-cylindrical, (15–)20–75(–80) × (1.5–)2–4(–5) μm, (1–)2–6(–8)-septate, occasionally slightly constricted at a few septa, subhyaline to pale olivaceous, thin-walled, smooth, apex obtuse in cylindrical conidia to subacute in above all obclavate conidia, base short obconically truncate, 1–2 μm wide, hila unthickened, not darkened.
Holotype: Cuba: Santiago de las Vegas, on Annona squamosa, 29 Dec. 1987, R. F. Castañeda Ruiz (HAL 1650 F). Isotype: INIFAT, C87/376.
Host range and distribution: On Annona (ˣatemoya [cherimola ͯ squamosa], muricata, purpurea, reticulata, squamosa, Annona sp.), Rollinia mucosa, Annonaceae, Asia (India, Karnataka; Japan, Taiwan), Central and South America (Brazil, Panama, Venezuela), West Indies (Cuba).
Note: Records of the present species on Asimina pygmaea [Annona pygmaea] probably refer to Pseudocercospora asiminae-pygmaeae.
Pseudocercospora annonarum (Petr. & Cif.) U. Braun & Crous, Mycotaxon 105: 212 (2008).
(Fig. 42)
Fig. 42.
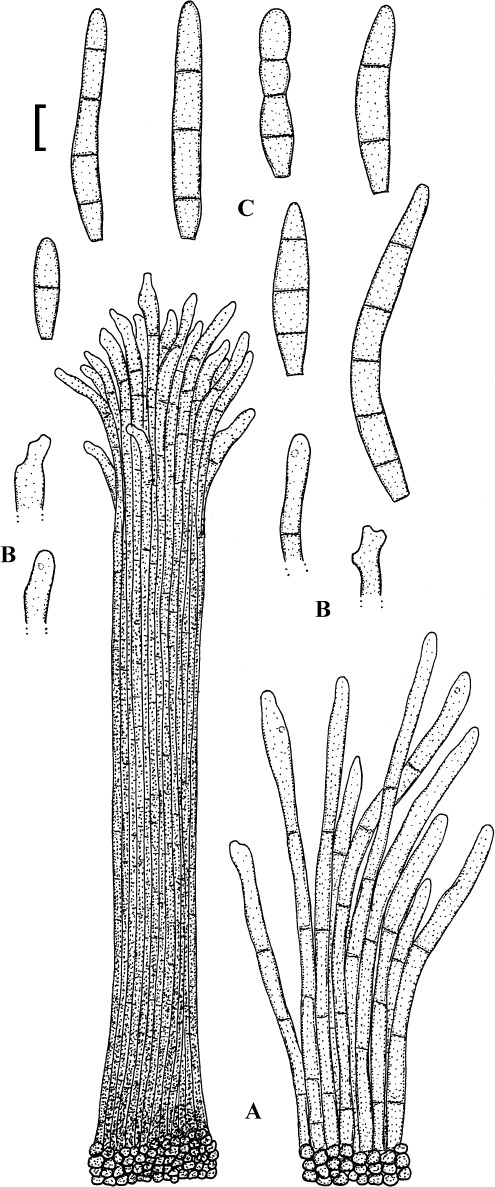
Pseudocercospora annonarum W Krypt. 1973-0010913, lectotype). A. Synnemata. B. Conidiophore tips. C. Conidia. Bar = 10 μm.
Basionym: Isariopsis annonarum Petr. & Cif., Ann. Mycol. 30: 345 (1932); as “anonarum”.
Synonyms: Phaeoisariopsis annonarum (Petr. & Cif.) U. Braun, Nova Hedwigia 50: 511 (1990).
Passalora annonarum (Petr. & Cif.) U. Braun & Crous, Mycosphaerella and Anam. 1: 438 (2003).
Illustrations: Braun (1990: 510, fig. 4e), Braun & Crous (2008: 213, fig. 3).
Description: Leaf spots amphigenous, subcircular to angular-irregular, 1–10 mm diam, occasionally confluent and larger, pale to dark brown, later greyish brown to dingy grey, on the lower leaf surface usually much paler, margin conspicuous, dark brown to blackish, narrow. Conidiomata synnematous, hypophyllous, scattered, rather inconspicuous to punctiform, brown to dark brown. Mycelium internal. Stromata immersed, 20–60 μm diam, brown. Synnemata 80–250 × 20–50 μm, brown, composed of a mostly firm subcylindrical stipe of numerous densely appressed parallel conidiophores and a more or less loose apical capitulum, stipe often somewhat wider at the very base, occasionally with conidiophores in dense fascicles, i.e. not distinctly synnematous, individual conidiophores 40–120 × 2–5 μm, terminal cells (conidiogenous cells) to 7 μm wide, olivaceous to medium brown, pluriseptate throughout, thin-walled, smooth; conidiogenous cells integrated, terminal, rarely intercalary or pleurogenous, 10–25(–30) μm long, conidiogenous loci inconspicuous to subconspicuous by being subdenticulate or by having an unthickened, but slightly darkened-refractive rim (paracercosporoid), visible in front view as minute circle, 1–2 μm diam. Conidia solitary, obclavate-cylindrical, occasionally subclavate, short conidia sometimes ellipsoid-ovoid, straight to curved, (15–)25–70(–80) × (3.5–)4–8(–9) μm, (0–)1–7(–10)-septate, occasionally constricted at the septa, pale to medium olivaceous or olivaceous brown, thin-walled (ca. 0.5 μm), smooth, apex obtuse, base obconically truncate to somewhat convex, (1.5–)2–2.5(–3) μm wide, hila unthickened, not darkened, at most somewhat refractive.
Lectotype (designated here, MycoBank, MBT204897): Dominican Republic: Santiago Province: Valle del Ciboa, on Annona squamosa, 22 Dec. 1930, E. L. Ekman (W Krypt. 1973-0010913). Isolectotype: S-F40467. Topotypes (1 Dec. 1930): NY 936880, 936881.
Host range and distribution: On Annona (cherimola, squamosa), Annonaceae, South America (Bolivia, Venezuela), West Indies (Dominican Republic).
Pseudocercospora annonifolii (Bat. & Peres) U. Braun & F. Freire, Bibl. Lichenol. 86: 82 (2003).
(Fig. 43)
Fig. 43.
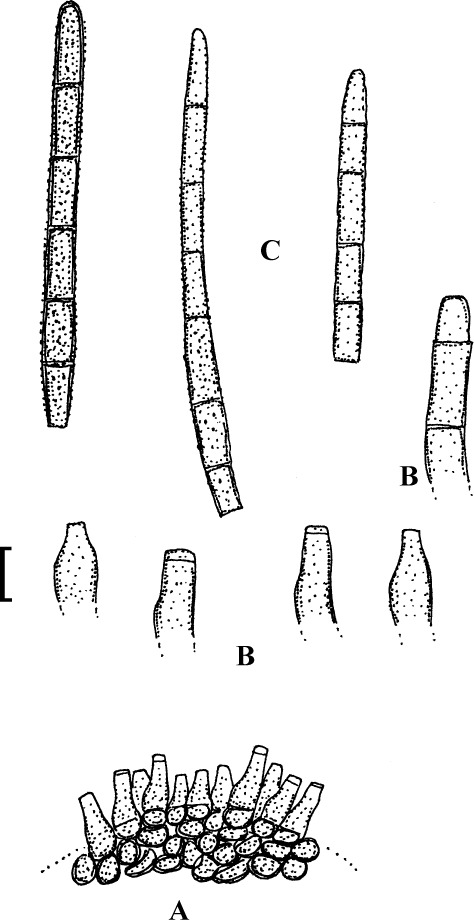
Pseudocercospora annonifolii (IMUR 21780, holotype). A. Sporodochial conidiophore fascicle. B. Conidiophores. C. Conidia. Bar = 10 μm.
Basionym: Cercospora annonifolii Bat. & Peres, Brotéria, Ser. Ci. Nat., 31: 372 (1962) [also in Anais do XIII Congresso da Sociedade Botânica do Brasil (Janeiro – 1962): 374 (1964)].
Literature: Crous & Braun (2003: 59), Braun & Crous (2008: 221).
Illustrations: Batista & Peres (1964: 375, fig. 96), Braun (2003: 93, fig. 4).
Description: Leaf spots amphigenous, subcircular, 2.5–5.5 mm diam, brown, surrounded by a narrow blackish brown marginal line. Caespituli (conidiomata) punctiform to almost pustulate, dark brown, scattered. Mycelium internal. Stromata immersed, sometimes erumpent, oblong in outline, 55–90 μm diam, brown. Conidiophores very numerous, in dense fascicles, arising from stromata, forming sporodochial conidiomata, erect, straight, subcylindrical-conical, ampulliform, 5–20(–30) × 3–8 μm, aseptate, olivaceous brown, thin-walled, smooth; conidiophores reduced to conidiogenous cells, unilocal, determinate or percurrently proliferating with 1(–2) fine annellations, conidiogenous loci 2–3.5 μm wide, unthickened, not darkened. Conidia solitary, cylindrical-vermiform (-obclavate), 20–240 × 3.5–5 μm, pluriseptate (with to 30 septa), olivaceous, thin-walled, verruculose, apex obtuse, rounded, base rounded to truncate, 2–3 μm wide, hila unthickened, not darkened.
Holotype: Brazil: Minas Gerais: Paraopeba, on Annona crassifolia, Annonaceae, 15 Aug. 1960, E. P. Heringer (IMUR 21780).
Host range and distribution: Only known from the type collection.
Notes: This species belongs morphologically to a group of Pseudocercospora spp. previously assigned to Cercostigmina. Species of this group are characterised by having sporodochial conidiomata and consistently unilocal, percurrent conidiogenous cells.
Pseudocercospora asiminae (Ellis & Morgan) U. Braun & Crous, Mycotaxon 105: 221 (2008).
(Fig. 44).
Fig. 44.
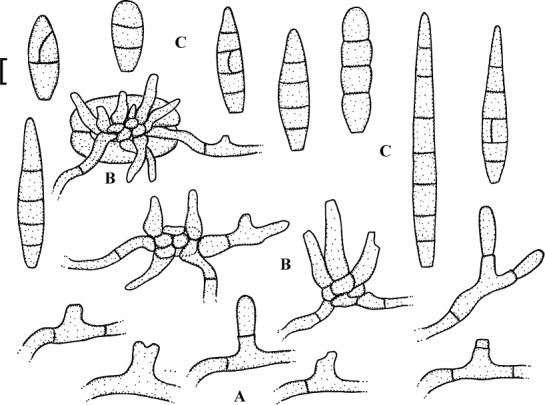
Pseudocercospora asiminae (NY 1097272, holotype). A. Solitary conidiophores arising from superficial hyphae. B. Conidiophore fascicles. C. Conidia. Bar = 10 μm.
Basionym: Phloeospora asiminae Ellis & Morgan, J. Mycol. 3: 88 (1887).
Synonyms: Rhopaloconidium asiminae (Ellis & Morgan) Petr., Sydowia 6: 301 (1952).
Cercospora asiminae Ellis & Kellerm., J. Mycol. 3: 103 (1887) [holotype: USA: Kansas: Mound City, on Asimina triloba, Jul. 1887, W. A. Kellerman (NY 985441)].
Centrospora asiminae (Ellis & Kellerm.) Deighton, Mycol. Pap. 124: 5 (1971).
Mycocentrospora asiminae (Ellis & Kellerm.) Deighton, Taxon 21: 716 (1972).
Miuraea asiminae (Ellis & Morgan) Arx & O. Constant., Proc. Kon. Ned. Akad. Wettensch., C 86: 39 (1983).
Literature: Saccardo (1892: 638), Chupp (1954: 45), Deighton (1971: 5), Braun (1995: 223), Crous & Braun (2003: 68).
Illustration: Braun (1995: 222, fig. 211a).
Exsiccatae: Barthol., Fungi Columb. 3244. Seym. & Earle, Econ. Fungi 102.
Description: Leaf spots amphigenous, angular-irregular, often vein-limited, 1–5 mm diam, at first pale brown, later often dull purplish violet to reddish brown or dark brown, margin indefinite, spots later sometimes confluent and larger. Caespituli hypophyllous, punctiform to subeffuse, whitish, loose to fairly dense, sometimes coalescent. Mycelium internal and external; internal hyphae branched, 1–4 μm wide, colourless, septate, sometimes forming loose stromatic hyphal aggregations, to 50 μm diam, substomatal to erumpent, almost superficial, hyaline to faintly pigmented; superficial hyphae arising from internal hyphae or substomatal to superficial hyphal aggregations, branched, septate, 2–5 μm wide, thin-walled, smooth, hyaline, subhyaline to pale yellowish, brownish or olivaceous. Conidiophores in small, loose fascicles, arising from substomatal to erumpent stromatic hyphal aggregations or solitary, arising from superficial hyphae, lateral, occasionally terminal, erect, straight, subcylindrical to slightly geniculate-sinuous, solitary conidiophores short, 6–20 × 4–10 μm, fasciculate conidiophores often somewhat longer, to 35 μm, aseptate, rarely with a single septum, hyaline to faintly pigmented, pale yellowish green, olivaceous or even brownish, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores usually reduced to conidiogenous cells, monoblastic, determinate to polyblastic, usually percurrently proliferating, occasionally sympodial, conidiogenous loci more or less truncate, unthickened, not darkened. Conidia solitary, straight to curved, shape variable, ellipsoid-ovoid, fusiform, obclavate, 20–80 × 4–12 μm, with 1–9 transverse septa and occasionally 1(–2) longitudinal or oblique septa, sometimes constricted at the septa, hyaline to faintly pigmented, pale yellowish, greenish, thin-walled, smooth, apex obtuse, base rounded to truncate, with broad hilum, unthickened, not darkened.
Holotype: USA: Ohio: Preston, on Asimina triloba, without date, A. P. Morgan 463 (NY 1097272).
Host range and distribution: On ?Annona squamosa, Asimina (parviflora, obovata, triloba, Asimina sp.), Annonaceae, North America (USA, Alabama, Florida, Illinois, Indiana, Iowa, Kansas, Maryland, Mississippi, Missouri, Ohio, Texas, West Virginia), ?Oceania (Niue).
Note: The record of this species on Annona squamosa from Niue (Dingley et al. 1981) is doubtful and needs verification.
Pseudocercospora asiminae-pygmaeae U. Braun, Monogr. Cercosporella, Ramularia Allied Genera (Phytopath. Hyphom.) 1: 223 (1995).
(Fig. 45).
Fig. 45.
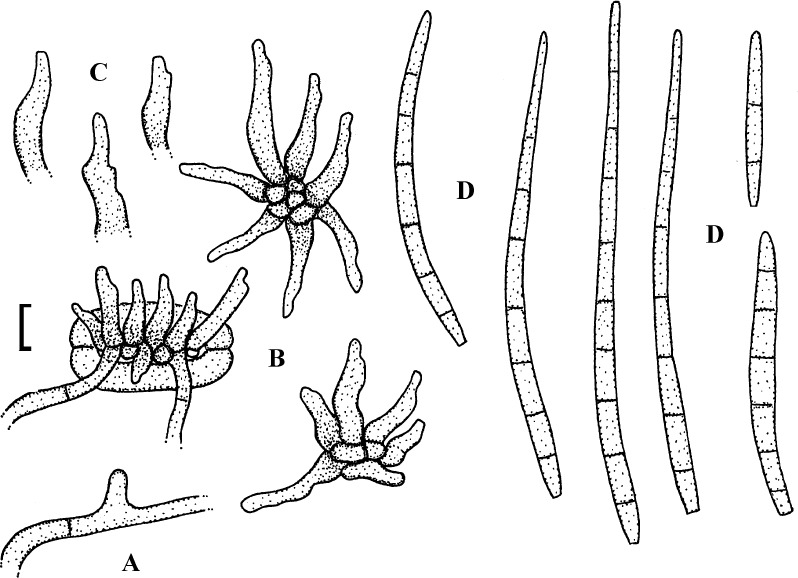
Pseudocercospora asiminae-pygmaeae (NY 985488, holotype). A. Superficial hypha with a solitary conidiophore. B. Conidiophore fascicles. C. Conidiophores. D. Conidia. Bar = 10 μm.
Illustration: Braun (1995: 222, fig. 211b).
Description: Leaf spots at first fairly inconspicuous, angular, vein-limited, 1–4 mm diam, dull greyish green, later dull brown, greyish to dark brown, subcircular to angular-irregular, to 8 mm diam. Caespituli hypophyllous, greyish white, sometimes darker, punctiform to subeffuse. Mycelium internal and external; internal hyphae branched, septate, thin-walled, smooth, forming small substomatal stromatic hyphal aggregations, yellowish brown, often erumpent; superficial hyphae sparingly to well-developed, arising from substomatal hyphal aggregations or through stromata, branched, septate, 2–5 μm wide, subhyaline to pale olivaceous, thin-walled, smooth. Conidiophores in small, loose fascicles, about 2–8, arising from internal hyphae or stromatic hyphal aggregations, through stomata or erumpent, or solitary, arising from superficial hyphae, erect, straight, subcylindrical or somewhat attenuated towards the tip to moderately geniculate-sinuous, unbranched, 10–40 × 3–6 μm, mostly aseptate, occasionally 1-septate, subhyaline to pale yellowish brown or olivaceous, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, 10–30 μm long, proliferation sympodial, conidiogenous loci unthickened, not darkened. Conidia solitary, obclavate-subcylindrical, rarely fusiform or subacicular, straight to curved, 25–140 × 3–6(–7) μm, 2–10-septate, subhyaline to very pale greenish or olivaceous, thin-walled, smooth, apex subacute, base truncate to obconically truncate, 2–2.5 μm wide, hila unthickened, not darkened.
Holotype: USA: Florida: Alachua County, La Crosse, on Asimina pygmaea, 17 Jul. 1936, G. F. Weber 11475 (NY 985488).
Host range and distribution: On Asimina (angustifolia, canescens, pygmaea), Annonaceae, North America (USA, Florida).
Pseudocercospora miliusae-tomentosae Kamal, Cercosporoid Fungi India: 197 (2010).
(Fig. 46).
Fig. 46.
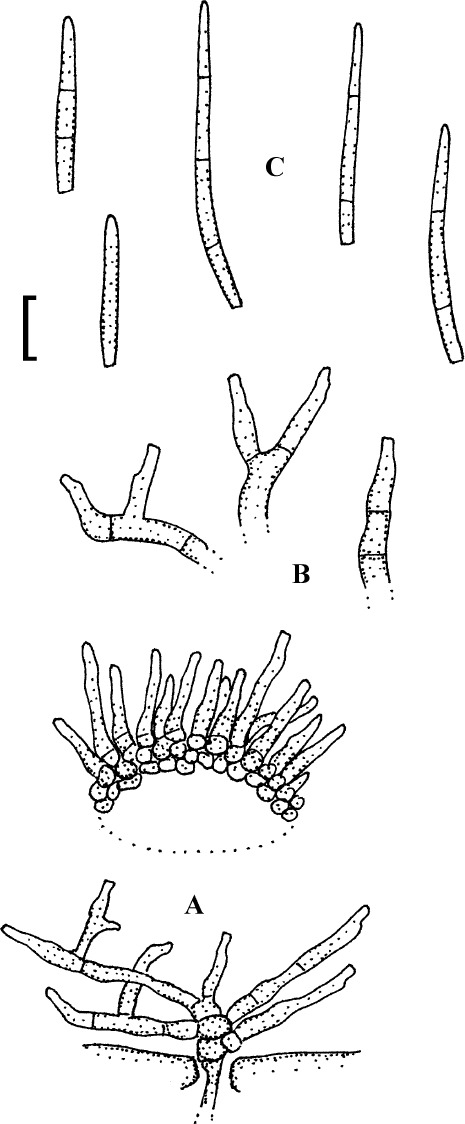
Pseudocercospora miliusae-tomentosae (based on Kamal 2010: 197, fig. 27). A. Conidiophore fascicles. B. Conidiophores. C. Conidia. Bar = 10 μm.
Synonym: Pseudocercospora miliusae Raghv. Singh et al., Mycotaxon 117: 140 (2011) [holotype: India: Uttar Pradesh: Gorakhpur, Vindhyavashini Park, on Miliusa tomentosa, Jan. 2008, R. Singh (HCIO 48786)].
Illustration: Kamal (2010: 197, fig. 27), Singh et al. (2011: 141, fig. 2).
Description: Leaf spots amphigenous, subcircular to angular-irregular, vein-limited, 1–21 mm diam, sometimes confluent, brown to blackish. Caespituli hypophyllous, effuse, brown. Mycelium internal. Stromata subepidermal, erumpent, about 15–40 μm diam, pseudoparenchymatous, brown. Conidiophores solitary or in small to moderately large fascicles, 2–50, arising from stromata, loose to dense, erumpent, erect to decumbent, straight, subcylindrical to usually geniculate-sinuous, unbranched or branched, 10–70 × 2–5 μm, 1–2-septate, light brown, wall somewhat thickened, smooth; conidiogenous cells integrated, terminal, 10–40 μm long, conidiogenous loci inconspicuous to subdenticulate, unthickened, not darkened. Conidia solitary, cylindrical-obclavate, straight to curved, 20–60 × 2–3 μm, 0–4-septate, pale brown, thin-walled, smooth, apex acute to subobtuse, base short obconically truncate, sometimes rounded, 1–1.5 μm wide, hila unthickened, not darkened.
Holotype: India: Uttar Pradesh: Gorakhpur, Vindhyavashini Park, on Miliusa tomentosa, Annonaceae, Jan. 2008, R. Singh (HCIO 48786).
Host range and distribution: Only known from the type collection.
Pseudocercospora oblecta (Syd.) Crous & U. Braun, Mycotaxon 105: 222 (2008).
(Fig. 47)
Fig. 47.
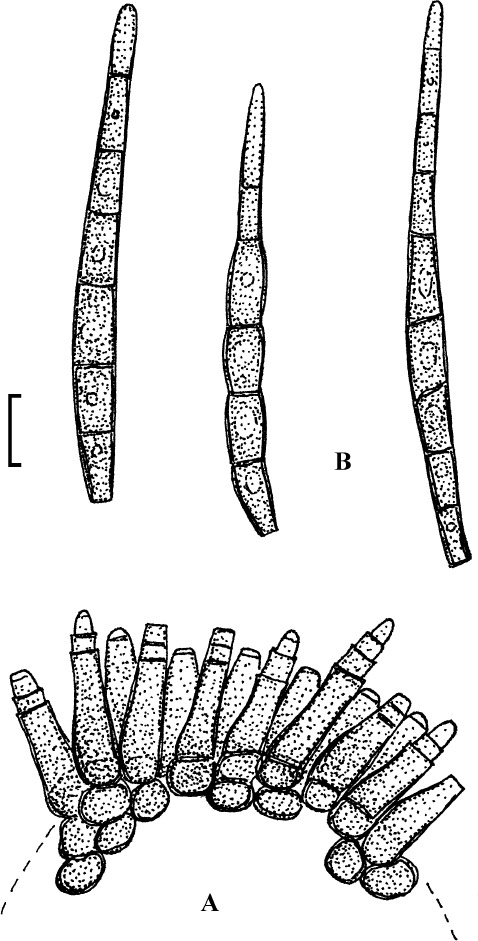
Pseudocercospora oblecta (CUP 40404, isolectotype). A. Sporodochial conidioma. B. Conidia. Bar = 10 μm.
Basionym: Cercospora oblecta Syd., Ann. Mycol. 33: 235 (1935).
Synonym: Stigmina oblecta (Syd.) Crous & U. Braun, Mycotaxon 57: 285 (1996).
Literature: Chupp (1954: 45), Crous & Braun (1996: 285), Crous & Braun (2003: 294).
Illustration: Crous & Braun (1996: 286, fig. 8).
Description: Leaf spots amphigenous, irregular, 2–6 mm diam, brown, grey-brown with age. Caespituli epiphyllous, punctiform-pustulate, dark. Mycelium internal. Stromata epiphyllous, well-developed, 40–90 μm diam, immersed, blackish brown to almost black. Conidiophores numerous, in dense fascicles, arising from stromata, forming sporodochial conidiomata, to 90 μm wide and 60 μm high, erumpent, erect, straight, cylindrical to somewhat irregularly shaped, unbranched, (10–)30–50(–60) × 3–7 μm, 0–2-septate, pale to medium olivaceous or brown, thin-walled, verruculose; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, subcylindrical or tapering towards a rounded to truncate tip, unilocal, proliferation percurrent, with 1–4 conspicuous annellations, conidiogenous loci unthickened and not darkened. Conidia solitary, obclavate to cylindrical, straight to curved, 40–120 × 4–8 μm, 1–15-septate, brown, olivaceous towards the apex, wall thin, verruculose, apex rounded, base obconically truncate or subtruncate, hila neither thickened nor darkened.
Lectotype (designated here, MycoBank, MBT204898): South Africa: Mpumalanga: Barberton, on Annona senegalensis, Apr. 1931, L. C. C. Liebenberg 26043 (PREM 26043). Isolectotypes: CUP 40404, 40405; K(M) IMI 37998.
Host range and distribution: On Annona (senegalensis, Annona sp.), Annonaceae, South Africa (Transvaal), South America (Brazil).
Notes: This species has been recorded from Brazil on Annona sp. (Mendes et al. 1998). Material was not available for confirmation of the correct identification.
Pseudocercospora polyalthiae J.M. Yen et al., Mycotaxon 16: 43 (1982).
(Fig. 48)
Fig. 48.
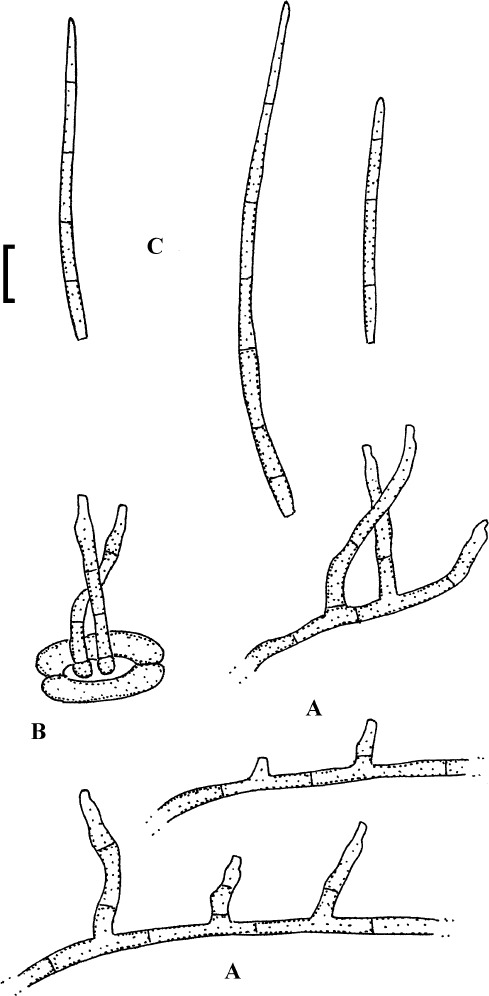
Pseudocercospora polyalthiae (based on Yen et al. 1982: 44, fig. 4 E–G). A. Solitary conidiophores arising from superficial hyphae. B. Conidiophores emerging through a stoma. C. Conidia. Bar = 10 μm.
Literature: Braun & Crous (2008), Kamal (2010: 210).
Illustration: Yen et al. (1982: 44, fig. 4 E–G).
Description: Leaf spots indistinct or lacking. Caespituli hypophyllous, rather indistinct. Mycelium internal and external; superficial hyphae branched, septate, 1.5–3 μm wide, very pale olivaceous, thin-walled, smooth. Stromata not developed. Conidiophores solitary, arising from superficial hyphae, lateral, occasionally with a single or few conidiophores emerging through stomata, erect, straight, subcylindrical-conical to geniculate-sinuous, unbranched or only occasionally branched, 12–70 × 3.5–5 μm, 0–8-septate, pale olivaceous brown, paler towards the tip, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, conidiogenous loci inconspicuous, unthickened, not darkened. Conidia solitary, narrowly obclavate-cylindrical, straight to curved, 40–155 × 2.5–3.5 μm, 3–13-septate, pale olivaceous brown, thin-walled, smooth, apex obtuse to subacute, base short obconically truncate, about 1–2 μm wide, hila neither thickened nor darkened.
Holotype: India: West Bengal: 24-Parganas, Garia, on Polyanthia suberosa, Annonaceae, 15 Dec. 1979, B. K. Das, Pcc4095 = Yen # 10585 (UC).
Host range and distribution: Only known from the type collection.
Pseudocercospora scitula (Syd.) Deighton, Mycol. Pap. 140: 152 (1976).
(Fig. 49)
Fig. 49.
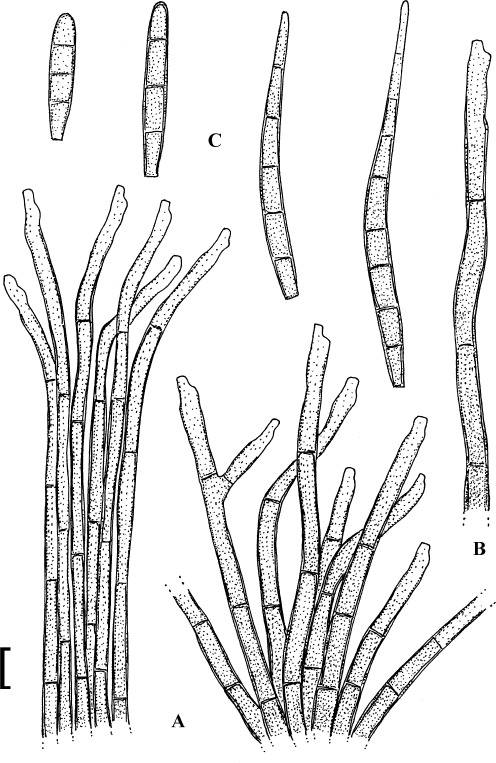
Pseudocercospora scitula (CUP 41178, isolectotype). A. Conidiophore fascicles (on the left synnematous). B. Conidiophore tip. C. Conidia. Bar = 10 μm.
Basionym: Cercospora scitula Syd., Ann. Mycol. 33: 236 (1935).
Literature: Chupp (1954: 46), Crous & Braun (1996: 308), Crous & Braun (2003: 369), Kamal (2010: 218).
Leaf spots formed as yellowish, ochraceous to dark brown blotches, to 50 mm diam, shape and size variable, margin indefinite. Caespituli hypophyllous, punctiform, medium dark brown to dark brown or formed as effuse, sooty colonies. Mycelium internal. Stromata lacking or almost so, only formed as aggregations of a few swollen hyphal cells. Conidiophores in small to moderately large fascicles, mostly 5–18, divergent to dense, sometimes almost coremioid, arising from internal hyphae or hyphal aggregations, through stomata, erect, straight, subcylindrical to distinctly geniculate-sinuous, above all in the upper half, often wider near the tip, unbranched or occasionally branched, 50–220 μm long, 3–6 μm wide below and 4–8 μm wide above, pluriseptate throughout, distance between septa 15–45 μm, pale to medium dark olivaceous brown or brown throughout or paler towards the tip, wall slightly thickened, to 1 μm, above all in the lower half, smooth; conidiogenous cells integrated, terminal, 20–50 μm long, proliferation sympodial, conidiogenous loci inconspicuous, visible as truncate tips or subdenticulate, 2–2.5 μm wide, but unthickened and not darkened. Conidia solitary, obclavate-cylindrical, occasionaly subclavate, straight to curved or sometimes sigmoid, 25–110 × 5–8 μm, 3–9-septate, pale to medium dark olivaceous or olivaceous brown, wall thin (< 1 μm), smooth, apex obtuse or conidia partly almost rostrate, base short to long obconically truncate, 2–2.5(–3) μm wide, hila unthickened, not darkened.
Lectotype (designated here, MycoBank, MBT204899): South Africa: Mpumalanga: Barberton, Nelspruit Research Station, on Annona senegalensis, Apr. 1931, L. C. C. Liebenberg 26027 (PREM 26027). Isolectotype: CUP 41178.
Host range and distribution: On Annona (senegalensis, Annona sp.), Annonaceae, Asia (India), Africa (Ghana, South Africa).
Pseudocercospora xenoannonicola Crous & Bench., Sydowia 52: 88 (2001).
(Fig. 50)
Fig. 50.
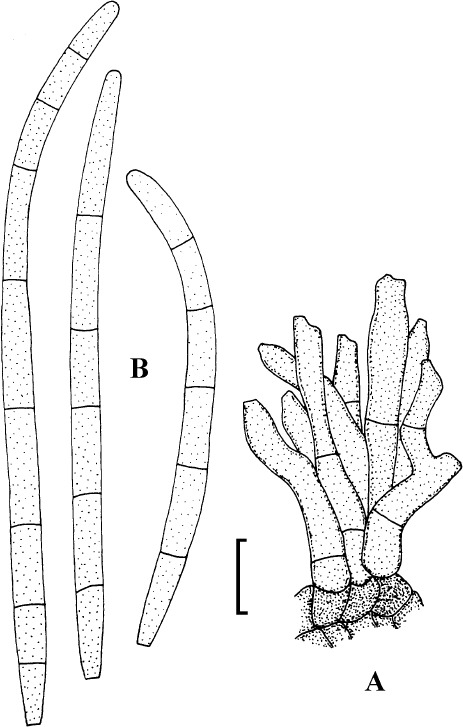
Pseudocercospora xenoannonicola (PREM 56555, holotype). A. Conidiophore fascicle. B. Conidia. Bar = 10 μm.
Illustration: Crous et al. (2001: 87, fig. 10).
Description: Leaf spots amphigenous, subcircular to irregular, 7–40 mm diam, initially formed as chlorotic yellowish discolorations, centre later grey to pale brown, surrounded by a dark brown outer region. Caespituli mainly hypophyllous, grey to medium brown, punctiform. Mycelium internal and external; superficial hyphae branched, septate, 2–3 μm wide, pale brown, thin-walled, smooth. Stromata to 30 μm diam, brown. Conidiophores in dense fascicles, to 60 μm wide and 35 μm high, straight, subcylindrical to geniculate-sinuous, unbranched, 10–30 × 3–4 μm, 0–2-septate, medium brown, paler towards the tip, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, 7–20 μm long, conidiogenous loci inconspicuous or visible as truncate tips, but always unthickened and not darkened. Conidia solitary, narrowly obclavate to subcylindrical, straight to curved, (30–)50–80(–100) × 2–3 μm, 5–7-septate, pale brown, thin-walled, smooth, apex obtuse to subacute, base short obconically truncate, 1–1.5 μm wide, hila unthickened, not darkened.
In vitro (MEA, CLA): Colonies olivaceous-grey, reverse olivaceous-black, sectoring, with uneven, smooth margins and sparse aerial mycelium, reaching 18–20 mm diam after 14 d at 25°C in the dark.
Holotype: Brazil: Pará: Belém, on Annona montana, Mar. 1998, R. L. Benchimol & F. C. Albuquerque (PREM 56555).
Host range and distribution: Only known from the type collection.
Zasmidium
Key to Zasmidium species on Annonaceae
1 Stromata 10–40 μm diam; conidiophores 20–150 μm long, pluriseptate; conidia subcylindrical-filiform, acicular to narrowly obclavate, pale olivaceous, yellowish brown to brown; on Annona spp. and an unknown host of the Annonaceae, Brazil .............................................................................. Z. annonaceae
Stromata lacking; conidiophores shorter, 17–75.5 μm, only 1–4-septate; conidia narrowly obclavate-cylindrical, pale olivaceous; on Polyalthia suberosa, India ..................... Z. polyalthiae
Zasmidium annonaceae (Henn.) U. Braun, Schlechtendalia 20: 100 (2010).
(Fig. 51)
Fig. 51.
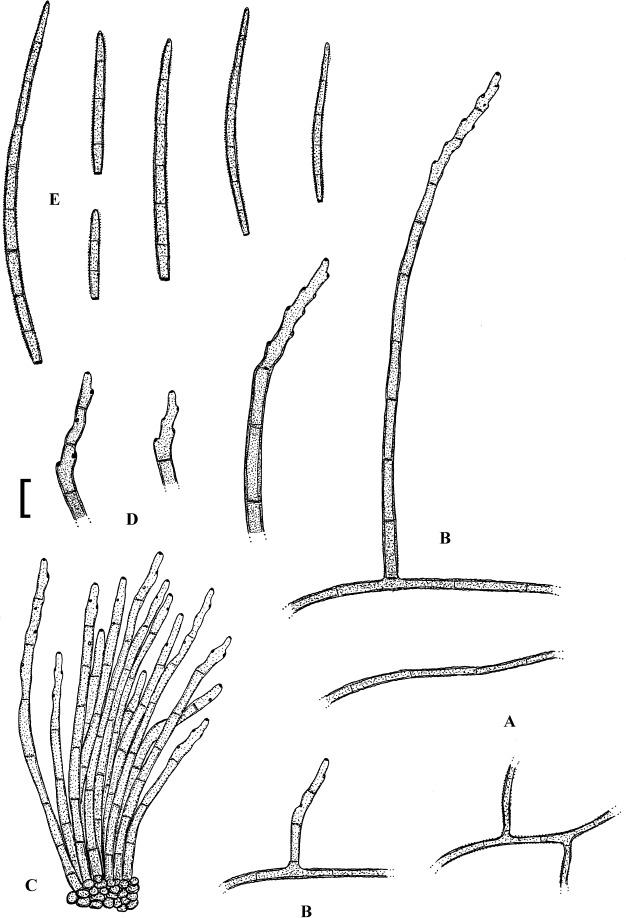
Zasmidium annonaceae (LEP, lectotype). A. Superficial hyphae. B. Solitary conidiophores arising from superficial hyphae. C. Conidiophore fascicle. D. Conidiophore tips. E. Conidia. Bar = 10 μm.
Basionym: Cercospora annonaceae Henn., Hedwigia 48: 18 (1909); “anonaceae”.
Synonym: Stenella annonaceae (Henn.) U. Braun, Mikol. Fitopatol. 30: 3 (1996).
Literature: Saccardo (1931: 868), Chupp (1954: 44), Crous & Braun (2003: 59), Braun & Crous (2008: 208).
Illustration: Braun & Mel’nik (1996: 2, fig. 2), Braun & Crous (2008: 210, fig. 1)
Description: Leaf spots amphigenous, subcircular to angular irregular, 1–25 mm diam, sometimes confluent, pale to dull dark brown, later greyish to dingy greyish white, margin indefinite or with a narrow darker border or marginal line. Colonies amphigenous, mainly hypophyllous, inconspicuous, effuse to punctiform, brown to greyish brown. Mycelium internal and external; superficial hyphae emerging through stomata, 1.5–3 μm wide, branched, septate, subhyaline, pale to medium olivaceous, olivaceous brown or yellowish brown, thin-walled, verruculose. Stromata absent or small, not very conspicuous, 10–40 μm diam, immersed, brown. Conidiophores solitary, arising from superficial hyphae, lateral, or in small to moderately large fascicles, loose to rather dense, sometimes almost coremioid, arising from internal hyphae or stromata, erect, unbranched, subcylindrical-filiform, straight to somewhat flexuous-sinuous, upper fertile part usually geniculate-sinuous, sometimes strongly so, 20–150 × (2.5–)3–4(–4.5) μm, pluriseptate throughout, pale to dark brown, yellowish brown or olivaceous brown, smooth, wall thin to slightly thickened (< 1 μm); conidiogenous cells integrated, terminal and intercalary, 5–20 μm long, occasionally longer, with several conspicuous conidiogenous loci, 1–1.5 μm diam, slightly thickened and darkened. Conidia solitary, subcylindrical-filiform, acicular, narrowly obclavate, straight to curved, 20–100 × 2.5–4.5 μm, 2–8-septate, pale olivaceous, yellowish brown to brown, verruculose, wall thin (about 0.5 μm), apex obtuse to subacute, base truncate to usually obconically truncate, 1–1.5(–2) μm wide, hila slightly thickened and darkened.
Lectotype (designated by Braun & Crous 2008): Brazil: São Paulo: Ague Branca, on an unidentified annonaceous host (probably Annona sp.), May 1903, Puttemans 738 (LEP).
Host range and distribution: On Annona (cherimola, reticulate, squamosa, Annona sp.), Annonaceae; North America (Mexico), South America (Brazil, Colombia, Ecuador, Venezuela), West Indies (Cuba).
Notes: “Pará, May 1903, Puttemans” is written on the original label of the corresponding sample deposited at LEP. This is undoubtedly type material since date and collector agree with the data given in the protologue. “Pará” is, however, incorrect since the material concerned had been collected in the São Paulo region as all other specimens that Hennings obtained from Puttemans. Additional material from Brazil on A. reticulata has been examined (Fungos de Minas Geraea, Viçosa-Escola, 23 Mar 1930, A. S. Muller, CUP-MG 157). Syntype material is not preserved at B.
Zasmidium polyalthiae (S. Chaudhary et al.) Kamal, Cercosporoid Fungi India: 246 (2010); as “polylathiae”.
(Fig. 52)
Fig. 52.
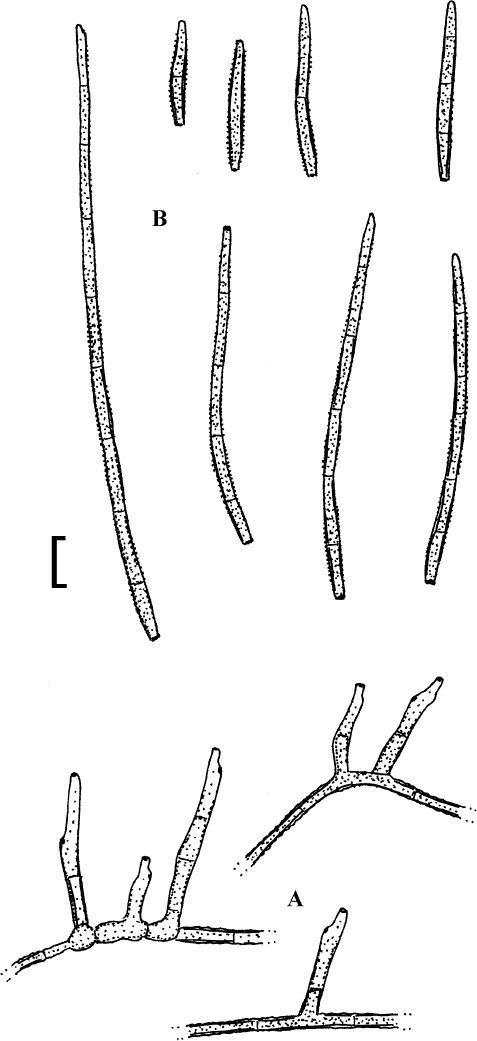
Zasmidium polyalthiae (based on Chaudhary et al. 2002: 58, fig. 2). A. Solitary conidiophores arising from superficial hyphae. B. Conidia. Bar = 10 μm.
Basionym: Stenella polyalthiae S. Chaudhary et al., Indian Phytopathol. 55: 58 (2002).
Illustration: Chaudhary et al. (2002: 58, fig. 2).
Description: Leaf spots hypophyllous, circular to subcircular, blackish, scattered. Colonies hypophyllous, punctiform. Mycelium internal and external; superficial hyphae branched, septate, 1.5–3 μm wide, subhyaline, faintly verruculose, thin-walled. Stromata absent. Conidiophores solitary, arising from superficial hyphae, lateral or terminal, erect, straight, subcylindrical to flexuous, geniculate-sinuous, unbranched or branched, 17–77.5 × 2.5–4 μm, 1–4-septate, brown, thin-walled, smooth; conidiogenous cells integrated, terminal or conidiophores sometimes reduced to conidiogenous cells, conidiogenous loci conspicuous, somewhat thickened and darkened. Conidia solitary, rarely catenate, narrowly obclavate-cylindrical, straight to curved, 20–157 × 2–3.5 μm, 0–8-septate, pale olivaceous, thin-walled, verruculose, apex obtuse, rounded, base obconically truncate, hila somewhat thickened and darkened.
Holotype: India: Uttar Pradesh: Maharajganj, Nichaul Forest, on Polyalthia suberosa, Annonaceae, Mar. 1990, S. Chaudhary (HCIO 43694). Isotype: GPU 9016.
Host range and distribution: Only known from the type collection.
ACKNOWLEDGEMENTS
We are very grateful to the directors and curators of B, BPI, CUP, HMAS, K, L, LE, LEP, LPS, NY, NYS, RREM, S, TNS, and W for loaning type material and other collections in their keeping during the course of monographic studies of cercosporoids fungi on the hosts of the families treated in part 5.
REFERENCES
- Alfieri SA, jr, Langdon KR, Wehburg C, Kimbrough JW. (1984) Index of plant diseases of Florida (revised). Florida Department of Agriculture and Consumer Services, Division of Plant Industry, Bulletin 11: 1–389. [Google Scholar]
- Aptroot A. (2006) Mycosphaerella and its Anamorphs: 2. Conspectus of Mycosphaerella. [CBS Biodiversity Series no. 5.] Utrecht: Centraalbureau voor Schimmelcultures. [Google Scholar]
- Bakhshi M, Arzanlou M, Babai-ahari A, Groenewald JZ, Braun U, Crous PW. (2014) Application of the consolidated species concept to Cercospora spp. from Iran. Persoonia 34: 65–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhshi M, Arzanlou M, Babai-ahari A, Groenewald JZ, Crous PW. (2015) Is morphology in Cercospora a reliable reflection of generic affinity? Phytotaxa 213(1): 022–034. [Google Scholar]
- Batista AC, Peres GEP. (1964) Alguns fungos Cercospora de Minas Gerais. Anais do XII Congresso da Sociedade Botânica do Brasil (Janeiro – 1962): 375–384. [Google Scholar]
- Bharadwaj LN, Sharma RC. (1994) Some new fungal diseases of Pistacia integerrima from Himachal Pradesh. Indian Forester 120: 545–547. [Google Scholar]
- Braun U. (1990) Studies on Ramularia and allied genera (III). Nova Hedwigia 50: 499–521. [Google Scholar]
- Braun U. (1995a) A Monograph of Cercosporella, Ramularia and allied genera (phytopathogenic hyphomycetes). Vol. 1 Eching: IHW-Verlag. [Google Scholar]
- Braun U. (1995b) Miscellaneous notes on phytopathogenic hyphomycetes (II). Mycotaxon 55: 223–241. [Google Scholar]
- Braun U. (1998) A Monograph of Cercosporella, Ramularia and allied genera (phytopathogenic hyphomycetes). Vol. 2 Eching: IHW-Verlag. [Google Scholar]
- Braun U. (2000) Annotated list of Cercospora spp. described by C. Spegazzini. Schlechtendalia 5: 57–79. [Google Scholar]
- Braun U. (2003) Miscellaneous notes on some cercosporoid hyphomycetes. Bibliotheca Lichenologica 86: 79–98. [Google Scholar]
- Braun U, Crous PW. (2006) (1732) Proposal to conserve the name Pseudocercospora against Stigmina and Phaeoisariopsis (Hyphomycetes). Taxon 55: 803. [Google Scholar]
- Braun U, Crous PW. (2008) Cercosporoid hyphomycetes on hosts of the Annonaceae: Cercospora annonaceae and Isariopsis annonarum revisited. Mycotaxon 105: 207–224. [Google Scholar]
- Braun U, Freire FCO. (2003) [“2002”] Some cercosporoid hyphomycetes from Brazil – II. Cryptogamie, Mycologie 23: 295–328. [Google Scholar]
- Braun U, Freire FCO. (2004) Some cercosporoid hyphomycetes from Brazil – III. Cryptogamie, Mycologie 25: 221–244. [Google Scholar]
- Braun U, Mel’nik VA. (1996) An annotated list of Cercospora type samples deposited in the herbarium of All-Russian Institute for Plant Protection (LEP). Mikologiya i Fitopatologiya 30(4): 1–9. [Google Scholar]
- Braun U, Mel’nik VA. (1997) Cercosporoid fungi from Russia and adjacent countries. Trudy Botanicheskogo Instituta Imeni V.L. Komarova, Rossijskaya Akademiya Nauk St Petersburg 20: 1–130. [Google Scholar]
- Braun U, Urtiaga R. (2008) New species and new records of cercosporoid hyphomycetes from Venezuela. Feddes Repertorium 119: 484–506. [Google Scholar]
- Braun U, Urtiaga R. (2013) New species and new records of cercosporoid hyphomycetes from Cuba and Venezuela (Part 2). Mycosphere 4: 165–205. [Google Scholar]
- Braun U, Crous PW, Nakashima C. (2014) Cercosporoid fungi (Mycosphaerellaceae) 2. Species on monocots (Acoraceae to Xyridaceae, excluding Poaceae). IMA Fungus 5: 203–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Crous PW, Nakashima C. (2015a) Cercosporoid fungi (Mycosphaerellaceae) 3. Species on monocots (Poaceae, true grasses). IMA Fungus 6: 25–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Crous PW, Nakashima C. (2015b) Cercosporoid fungi (Mycosphaerellaceae) 4. Species on dicots (Acanthaceae to Amaranthaceae). IMA Fungus 6: 373–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Crous PW, Pons N. (2002) Annotated list of Cercospora species (epithets a-b) described by C. Chupp. Feddes Repertorium 113: 112–127. [Google Scholar]
- Braun U, David J, Freire FCO. (1999) Some cercosporoid hyphomycetes from Brazil. Cryptogamie, Mycologie 20: 95–106. [Google Scholar]
- Braun U, Mouchacca J, McKenzie EHC. (1999) Cercosporoid hyphomycetes from New Caledonia and some other Pacific Islands. New Zealand Journal of Botany 37: 297–327. [Google Scholar]
- Braun U, Nakashima C, Crous PW. (2013) Cercosporoid fungi (Mycosphaerellaceae) 1. Species on other fungi, Pteridophyta and Gymnospermae. IMA Fungus 4: 265–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridson GDR. (2004a) BPH–2, Periodicals with Botanical Content, constituting a second edition of Botanico-Periodicum-Huntianum. Vol. 1, A–M. Pittsburgh: Hunt Institute for Botanical Documentation. [Google Scholar]
- Bridson GDR. (2004b) BPH–2, Periodicals with Botanical Content, constituting a second edition of Botanico-Periodicum-Huntianum. Vol. 2, N–Z. Pittsburgh: Hunt Institute for Botanical Documentation. [Google Scholar]
- Brummitt RK, Powell CE. (1992) Authors of Plant Names. Kew: Royal Botanic Gardens. [Google Scholar]
- Castañeda Ruiz RF, Braun U. (1989) Cercospora and allied genera of Cuba (I). Cryptogamic Botany 1: 42–55. [Google Scholar]
- Castellani E, Casulli F. (1981) Cashew leaf spot disease caused by Pseudocercospora anacardii. Rivista di Agricoltura Subtropicale e Tropicale 75: 101–105. [Google Scholar]
- Chaudhary S, Sharma N, Kamal (2002) Three new species of Stenella. Indian Phytopathology 55: 57–60. [Google Scholar]
- Chupp C. (1954) A Monograph of the fungus genus Cercospora. Ithaca: C. Chupp. [Google Scholar]
- Crous PW, Braun U. (1994) Cercospora species and similar fungi occurring in South Africa. Sydowia 46: 204–224. [Google Scholar]
- Crous PW, Braun U. (1996). Cercosporoid fungi from South Africa. Mycotaxon 57: 233–321. [Google Scholar]
- Crous PW, Braun U. (2001) A reassessment of the Cercospora species described by C. Chupp: specimens deposited at BPI, Maryland, U.S.A. Mycotaxon 78: 327–343. [Google Scholar]
- Crous PW, Braun U. (2003) Mycosphaerella and its Anamorphs: 1. Names published in Cercospora and Passalora. [CBS Biodiversity Series no. 1]. Utrecht: Centraalbureau voor Schimmelcultures. [Google Scholar]
- Crous PW, Berchimol RL, Albuquerque FC, Alfenas AC. (2001) Foliicolous anamorphs of Mycosphaerella from South America. Sydowia 52: 78–91. [Google Scholar]
- Crous PW, Braun U, Hunter GC, Wingfield MJ, Verkley GJM, et al (2013) Phylogenetic lineages in Pseudocercospora. Studies in Mycology 75: 37–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Wingfield MJ, Wood AR, Shin HD, et al (2009a) Phylogeny and taxonomy of obscure genera of microfungi. Persoonia 22: 139–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Groenewald M, Caldwell P, Braun U, Harrington TC. (2006) Species of Cercospora associated with grey leaf spot of maize. Studies in Mycology 55: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, et al (2009b) Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Shivas RG, Quaedvlieg W, van der Bank M, Zhang Y, et al (2014) Fungal Planet description sheets: 214–280. Persoonia 32: 184–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Hunter GC, et al (2009c) Unravelling Mycosphaerella: do you believe in genera? Persoonia 23: 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Shivas RG, Burges TI, Decock CA, et al (2012) Fungal Planet description sheets: 138–182. Persoonia 28: 138–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deighton FC. (1971) Studies on Cercospora and allied genera. III. Centrospora. Mycological Papers 124: 1–13. [Google Scholar]
- Deighton FC. (1976) Studies on Cercospora and allied genera. VI. Pseudocercospora Speg., Pantospora Cif. and Cercoseptoria Petr. Mycological Papers 140: 1–168. [Google Scholar]
- Deighton FC. (1979) Studies on Cercospora and allied genera. VII. New species and redispositions. Mycological Papers 144: 1–56. [Google Scholar]
- Dharkar N, Subhedar A. (2006) Two rare synnematous fungi from Wardha District. Geobios 33: 206–208. [Google Scholar]
- Dingley JM, Fullerton RA, McKenzie EHC. (1981) Survey of agricultural pests and diseases. Records of fungi, bacteria, algae, and angiosperms pathogenic on plants in Cook Islands, Fiji, Kiribati, Niue, Tonga, Tuvalu, and Western Samoa. F.A.O., Technical Report 2: 1–485. [Google Scholar]
- Ellis MB. (1971) Dematiaceous Hyphomycetes. Kew: Commonwealth Mycological Institute. [Google Scholar]
- Ellis MB. (1976) More Dematiaceous Hyphomycetes. Kew: Commonwealth Mycological Institute. [Google Scholar]
- Groenewald JZ, Nakashima C, Nishikawa J, Shin HD, Park JH, et al. (2013) Species concepts in Cercospora: spotting the weeds among the roses. Studies in Mycology 75: 115–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guatimosim E, Schwartsburd PB, Barreto RW, Crous PW. (2016) Novel fungi from an ancient niche. Cercosporoid and related sexual morphs on ferns. Persoonia 37: 106–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YL. (1986) Studies on hyphomycetes of Shennongjia. I. Cercospora, Cercosporidium and Mycovellosiella. Acta Mycologica Sinica, Supplementum 1: 334–341. [Google Scholar]
- Guo YL, Hsieh WH. (1995) The genus Pseudocercospora in China. Mycosystema, Monographicum Series 2: 1–388. [Google Scholar]
- Guo YL, Jiang Y. (2000) Studies on Cercospora and allied genera in China I. Mycotaxon 74: 257–266. [Google Scholar]
- Guo YL, Liu XJ, Hsieh WH. (1998) Pseudocercospora. [Flora Fungorum Sinicorum vol. 9] Beijing: Science Press. [Google Scholar]
- Guo YL, Liu XJ, Hsieh WH. (2003) Mycovellosiella, Passalora, Phaeoramularia. [Flora Fungorum Sinicorum vol. 20] Beijing: Science Press. [Google Scholar]
- Guo YL, Liu XJ, Hsieh WH. (2005) Cercospora. [Flora Fungorum Sinicorum vol. 24] Beijing: Science Press. [Google Scholar]
- Hsieh WH, Goh TK. (1990) Cercospora and similar Fungi from Taiwan. Taipei: Maw Chang Book Company. [Google Scholar]
- Hyde KD. (1992) Stigmina mangiferae. IMI Descriptions of Fungi and Bacteria 1130: 1–2. [Google Scholar]
- Inácio CA, Dianese JC. (1999) A new Mycovellosiella species on Myracrodruon urunduva. Mycotaxon 72: 253–263. [Google Scholar]
- Kakoti RK, Saikia UN, Lamar D. (1998) Phytopathogenic fungi of north-east India – IV. Annals of Agri Bio Research 3: 207–210. [Google Scholar]
- Kamal (2010) Cercosporoid Fungi of India. Dehra Dun: Bishen Singh Mahendra Pal Singh. [Google Scholar]
- Kamal, Naraiyan P. (1986) Fungi from hilly tracts of Uttar Pradesh – I. Indian Phytopathology 39: 198–203. [Google Scholar]
- Kamal, Narayan P, Verma RP. (1987) [“1986“] Fungi from hilly tracts of UP – II. Indian Phytopathology 39: 453–458. [Google Scholar]
- Katsuki S. (1965) Cercosporae of Japan. Transactions of the Mycological Society of Japan, Extra Issue 1: 1–100. [Google Scholar]
- Katsuki S, Kobayashi T. (1976) Cercosporae of Japan and allied genera (Supplement 4). Transactions of the Mycological Society of Japan 17: 272–279. [Google Scholar]
- Kumar P, Kamal (1982) A new species of Mycovellosiella from India. Current Science 51: 846–847. [Google Scholar]
- McNeill J, Barrie FR, Buck WR, Demoulin V, Greuter W. et al (2012) International Code of Nomenclature for algae, fungi, and plants (Melbourne Code) adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011 [Regnum Vegetabile, 154.] Königstein: Koeltz Scientific Books. [Google Scholar]
- Mehrotra MD, Verma RK. (1991) Some new hyphomycetes associated with leaf spots of trees in India. Mycological Research 95: 1163–1168. [Google Scholar]
- Mendes MAS, da Silva VL, Dianese JC, Ferreira MASV, dos Santos CEN. et al (1998) Fungos em Plants no Brasil. Brasilia: Embrapa-SPI/Embrapa-Cenargen. [Google Scholar]
- Munjal RL, Lall G, Chona BL. (1962) [“1961”] Some Cercospora species from India – VI. Indian Phytopathology 14: 179–190. [Google Scholar]
- Nakashima C, Tanda S, Kobayashi T. (2002) Addition and re-examination of Japanese species belonging to the genus Cercospora and allied genera. IV. Newly recorded species from Japan (1). Mycoscience 43: 95–102. [Google Scholar]
- Nguanhom J, Cheewangkoon R, Groenewald JZ, Braun U, To-Anun C, Crous PW. (2015) Taxonomy and phylogeny of Cercospora spp. from Northern Thailand. Phytotaxa 233(1): 27–48. [Google Scholar]
- O’Farrell P, Armour J, Reid D. (2002) The effect of nitrogene on cashew in north Queensland 1995–1999. Rural Industries Research and Development Corporation, Publication W02/001: 1–29. [Google Scholar]
- Phengsintham P, Braun U, McKenzie EHC, Chukeatirote E, Cai L, Hyde KD. (2013) Monograph of cercosporoid fungi from Thailand. Plant Pathology & Quarantine 3: 67–138. [Google Scholar]
- Phengsintham P, Chukeatirote E, McKenzie EHC, Moslem MA, Hyde KD, Braun U. (2012) Fourteen new records of cercosporoids from Thailand. Maejo International Journal of Science and Technology 6: 47–61. [Google Scholar]
- Phengsintham P, Chukeatirote E, McKenzie EHC, Hyde KD, Braun U. (2013) Monograph of cercosporoid fungi from Laos. Current Research in Environmental & Applied Mycology 3: 34–158. [Google Scholar]
- Rai AN, Kamal (1987) [“1986”] Fungi of Gorakhpur – XLII. Kavaka 14: 31–36. [Google Scholar]
- Rossman AY, Crous PW, Hyde KD, Hawksworth DL, Aptroot A. et al (2015) Recommended names for pleomorphic genera in Dothideomycetes. IMA Fungus 6: 507–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccardo PA. (1886) Sylloge Fungorum omnium hucusque cognitum. Vol. 4 Padova: P.A. Saccardo. [Google Scholar]
- Saccardo PA. (1892) Sylloge Fungorum omnium hucusque cognitum. Vol. 10 Padova: P.A. Saccardo. [Google Scholar]
- Saccardo PA. (1902) Sylloge Fungorum omnium hucusque cognitum. Vol. 16 [Saccardo PA, Sydow P, eds]. Padova: P.A. Saccardo. [Google Scholar]
- Saccardo PA. (1913) Sylloge Fungorum omnium hucusque cognitum, Vol. 22 [Saccardo PA, Trotter A, eds]. Padova: P.A. Saccardo. [Google Scholar]
- Saccardo PA. (1931) Sylloge Fungorum omnium hucusque cognitum. Vol. 25 [Trotter A, ed.]. Avellino: sumptis coherendum Saccardo Typis Pergola. [Google Scholar]
- Sarbajna KK. (1990) Two new species of Pseudocercospora from West Bengal. Indian Phytopathology 43: 20–25. [Google Scholar]
- Sarbajna KK, Chattopadhyay BK. (1991) Studies on the genus Pseudocercospora Speg. Journal of Mycopathological Research 29: 43–50. [Google Scholar]
- Shin HD, Kim JD. (2001) Cercospora and allied genera from Korea. Plant Pathogens of Korea 7: 1–303. [Google Scholar]
- Siboe GM, Kirk PM, David JC, Cannon PF. (2000) Necrotrophic fungi from Kenyan endemic and rare plants. Sydowia 52: 286–304. [Google Scholar]
- Singh S, Kamal (1978) [“1977”] Fungi of Gorakhpur. VI. Indian Phytopathology 31: 315–317. [Google Scholar]
- Singh R, Kumar S, Kamal (2011) Two new species of Passalora and Pseudocercospora from northeastern Uttar Pradesh, India. Mycotaxon 117: 137–143. [Google Scholar]
- Sivanesan A. (1984) The Bitunicate Ascomycetes and their Anamorphs. Vaduz: J. Cramer. [Google Scholar]
- Teixeira LMS. (1988) Diseases. In: Cultura do cajueiro no Nordeste do Brazil (Lima VPMS, ed.): 156–179. Fortaleza: BNB-ETENE. [Google Scholar]
- Todawat Nawalsingh J, Papdiwal PB. (2011) Leaf spot diseases of some fruit trees of Aurangabad district, Maharashtra. Bioinfolet – A Quarterly Journal of Life Science 8: 87–90. [Google Scholar]
- Vasudeva RS. (1963) Indian Cercosporae. New Delhi: Indian Council of Agricultural Research. [Google Scholar]
- Verkley GJM, Crous PW, Groenewald JZ, Braun U, Aptroot A. (2004) Mycosphaerella punctiformis revisited: morphology, phylogeny, and epitypification of the type species of the genus Mycosphaerella (Dothideales, Ascomycota). Mycological Research 108: 1271–1282. [DOI] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Verkley GJM, Braun U, Crous PW. (2015) The rise of Ramularia from the Mycosphaerella labyrinth. Fungal Biology 119: 823–843. [DOI] [PubMed] [Google Scholar]
- Wijayawardene NN, Crous PW, Kirk PM, Hawksworth DL, Boonmee S. et al (2014) Naming and outline of Dothideomycetes – 2014 including proposals for the protection or suppression of generic names. Fungal Diversity 69: 1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen JM, Kar AK, Das BK. (1982) Studies on hyphomycetes from West Bengal, India, I. Cercospora and allied genera of West Bengal, 1. Mycotaxon 16: 35–57. [Google Scholar]


